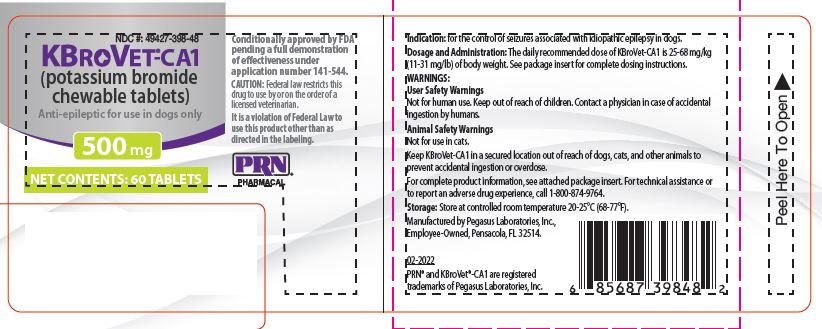
Label: KBROVET-CA1 500- potassium bromide tablet, chewable
- NDC Code(s): 49427-324-48, 49427-324-50, 49427-398-48, 49427-398-50
- Packager: Pegasus Laboratories, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Conditional New Animal Drug Application
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION:
- CONTRAINDICATIONS:
-
DESCRIPTION:
KBroVet-CA1 are flavored chewable tablets that contain potassium bromide (KBr). KBr is an odorless, colorless crystal or white crystalline powder or white granular solid with a pungent bitter saline taste. The molar mass of KBr is 119.002 g/mol, with a high soluability in water, glycerol and ethanol.
- INDICATION:
-
DOSAGE AND ADMINISTRATION:
The total recommended daily dosage range for oral administration is 25 - 68 mg/kg (11-31 mg/lb) of body weight. The dosage of KBroVet-CA1 should be adjusted based on monitoring of clinical response of the individual patient. KBroVet-CA1 may be dosed with or without food. Use of an initial loading dosage regimen may be considered on an individual patient basis, balancing the time required to achieve a therapeutic response while minimizing side effects.
- WARNINGS:User Safety Warnings
-
PRECAUTIONS:
Dogs receiving KBr should be carefully monitored when changing diets, administering chloride-containing IV fluids, and administering concurrent medications. Careful monitoring is important in dogs that have a condition that may cause difficulty maintaining electrolyte balance.
Animals with decreased renal function may be predisposed to bromide toxicosis.
Some dogs may experience epileptic episodes that are unresponsive or refractory to KBr monotherapy and KBr alone may not be adequate treatment for every dog with idiopathic epilepsy.
The safe use of KBroVet-CA1 has not been evaluated in dogs that are intended for breeding, or that are pregnant or lactating. The safe use of KBr in neonates and young animals has not been established. Reproductive effects of KBr have been reported in other species. In dogs, ataxia, diarrhea, hematochezia, excessive salivation, shivering, skin lesions, stupor progressing to coma and death have been reported with a dose of 200 to 500 mg/kg a day for 4 to 26 weeks.
-
ADVERSE REACTIONS:
In a retrospective field study of 51 dogs diagnosed with idio- pathic epilepsy and receiving only KBr to control seizures associated with idiopathic epilepsy, adverse reactions were documented for the initial 60 days of treatment. Increased appetite, weight gain, vomiting/regurgitation and sedation were the most common clinical abnormalities documented in the 60 day period after start of KBr therapy (Table 1).
Table 1. Adverse Reactions Reported During Initial Dosing Phase (60 Day Period After Start of KBr Therapy)
Adverse Reaction Number of Dogs with the
Adverse Reaction
Increased Appetite 11 Weight Gain 8 Vomiting 5 Regurgitation 4 Sedation 3 Polydipsia 2 Ataxia 2 Polyuria 2 Weakness 2 Decreased Activity 1 Diarrhea 1 Disorientation 1 Lethargy 1 Partial Lack of Efficacy 1 Petit Mal Epilepsy 1 Seizure 1 Tiredness 1 Tremors 1
Adverse reactions were also documented during the 30 days prior to KBr sample submission. Weight gain, weakness, ataxia, and increased appetite were the most common adverse reactions documented during this time period (Table 2).Table 2. Adverse Reactions Reported During Dosing Phase (30 Day Period Before KBr Sample Submission)
Adverse Reaction Number of Dogs with the
Adverse Reaction
Weight Gain 7 Weakness 5 Ataxia 4 Increased Appetite 4 Polydipsia 3 Sedation 3 Diarrhea 2 Polyuria 2 Regurgitation 2 Vomiting 2 Decreased Appetite 1 Disorientation 1 Loose Stool 1 Panting 1 Tremors 1 Adverse events associated with concurrent use of KBr with other antiepileptic drugs such as phenobarbital have been reported. Neurologic signs were the most common adverse event and included sedation, irritability, restlessness, depres- sion, behavioral changes, ataxia, hind limb paresis, mydriasis, stupor, and coma. The neurologic signs were reported to be reversible.
- CONTACT INFORMATION:
-
CLINICAL PHARMACOLOGY
Mechanism of action: KBr is a halide salt that is thought to exert its antiepileptic activity by passing through neuronal chloride ion channels, thereby hyperpolarizing neuronal membranes, raising the seizure threshold, and stabilizing neurons against excitatory input from epileptic foci.
Pharmacokinetics: The pharmacokinetics of a multi-dose regimen of administration in normal dogs have been evaluated as described in a comprehensive literature review. In one study, KBr was administered at 30 mg/kg orally every 12 hrs for a period of 115 days. Serum, urine, and cerebrospinal fluid (CSF) bromide concentrations were measured at the onset of dosing, during the accumulation phase, at steady-state, and after a subsequent dose adjustment. Median elimination half-life and steady-state serum concen- tration were 15.2 days and 245 mg/dL, respectively. Apparent total body clearance was 16.4 mL/day/kg and volume of distribution was 0.40 L/kg. The CSF:serum bromide ratio at steady-state was 0.77.
Distribution, Metabolism, and Elimination: Bromide distributes into the CSF and interstitial tissues of the brain and is actively transported out of the CNS via the choroid plexus. At pharmacological doses, the active transport mechanism is overwhelmed and bromide accumulates in the brain and CSF. Bromide is not metabolized by the liver and is eliminated unchanged, primarily by renal clearance. Increased dietary consumption of chloride can promote loss of bromide in the urine, leading to a lowering of serum bromide concentrations. Decreased chloride consumption will promote increased renal reabsorption of bromide, causing an increase in bromide elimination half-life in dogs.
-
REASONABLE EXPECTATION OF EFFECTIVENESS:
KBroVet-CA1 is conditionally approved pending a full demonstration of effectiveness.
Additional information for Conditional Approvals can be found by searching www.fda.gov for “animal conditional approval”.
Two retrospective studies were used to determine the dose and demonstrate a reasonable expectation of effectiveness for KBroVet-CA1 for the control of seizures associated with idiopathic epilepsy in dogs.
In a dose determination retrospective study, the total daily oral dose of KBr given for greater than or equal to 45 days (approaching steady- state conditions) was described. To be included in this study, cases were required to meet the following eligibility require- ments: samples submitted for serum bromide concentration evaluation within the required date range (January 1, 2003 to August 31, 2010), and dogs were between greater than or equal to 0.5 and less than or equal to 5.0 years of age, receiving only KBr to control seizures associated with idiopathic epilepsy, administered KBr once or twice daily for greater than or equal to 45 days at the dose noted on the submission form, and the serum bromide concentration was greater than or equal to 0.8 and less than or equal to 3.5 mg/mL.
A total of 284 case records (58.5% male and 41.6% female), with a mean age of 3.6 years (0.7-5.0 years) and a mean body weight of 20.5 kg (1.3-88.2 kg), were evaluated between January 1, 2003 to August 31, 2010. The mean total daily oral dose was 46.6 (±21.9) mg/kg with a range of 24.5-68.3 mg/kg. These results describe the total daily oral dose range to achieve serum bromide concentrations within 10% of the published therapeutic range (greater than or equal to 0.8 and less than or equal to 3.5 mg/mL)1,2 for dogs with idiopathic epilepsy.
A pilot retrospective study involving review of case records of 51 client-owned dogs was conducted to evaluate the effec- tiveness of KBr in dogs. This retrospective study evaluated case records of dogs previously receiving only KBr to control seizures associated with idiopathic epilepsy and for which blood samples had been analyzed to quantify serum bromide concentrations for the purpose of therapeutic drug monitoring.
Seizure counts, seizure count changes, seizure event days per month and seizure severity scores were tabulated for eligible cases, comparing the 30 day period before initial treatment with KBr and the 30 day period of steady state KBr dosing. Seizure count within an individual case was required to decrease by 50% or greater in order for the case to be classified as a seizure count success. Similarly, reduction in the number of seizure event days per month by greater than or equal to 50% was required for the case to be classified as a seizure event day count success. No increase in severity score denoted an individual case treatment success for this variable. Of the 51 evaluable cases, 27 were determined as valid for safety and effectiveness data and 24 were determined to be valid for only safety data.
Of the 27 cases, 19 (70%) were defined as “success” and 8 (30%) were defined as “failures” based on seizure count results. Eighteen (67%) were defined as “success” and 9 (33%) were defined as “failures” based on seizure event day results. Seizure severity score decreased or did not change in 25 of the 27 cases evaluated for effectiveness. Overall, of the 27 dogs included in the effectiveness analysis, 18 (67%) were considered treatment successes and 9 (33%) were considered treatment failures.
ANIMAL SAFETY:
Safety was assessed in a systematic review of literature and a retrospective field study. Reversible neurologic signs were the most consistently reported adverse effect and were generally associated with adjunctive KBr treatment or high serum bromide concentrations. Adverse effects were also seen in some dogs with low serum bromide concentration. Dermatologic and respiratory abnormalities were rare in dogs. Evidence suggested that administration of KBr with food may alleviate gastrointestinal irritation and that monitoring for polyphagia, thyroid hormone abnormalities, and high serum bromide concentrations may be beneficial.
-
HOW SUPPLIED:
KBroVet-CA1 are flavored, non-scored tablets containing
250 mg or 500 mg of potassium bromide per tablet. KBroVet-CA1 is packaged in bottles containing 60 or 180 tablets.500 mg Tablet (60 ct) bottle NDC 49427-398-48
250 mg Tablet (60 ct) bottle NDC 49427-397-48
500 mg Tablet (180 ct) bottle NDC 49427-398-50
250 mg Tablet (180 ct) bottle NDC 49427-397-50
- STORAGE CONDITIONS:
- REFERENCES
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KBROVET-CA1 500
potassium bromide tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:49427-324 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM BROMIDE (UNII: OSD78555ZM) (BROMIDE ION - UNII:952902IX06) POTASSIUM BROMIDE 500 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) 385.7 mg DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) 266 mg CYANOCOBALAMIN (UNII: P6YC3EG204) 0.013 mg STEARIC ACID (UNII: 4ELV7Z65AP) 121 mg Product Characteristics Color brown (tan;with;brown;speckles) Score no score Shape ROUND Size 16mm Flavor LIVER Imprint Code 500;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49427-324-48 60 in 1 PACKAGE 2 NDC:49427-324-50 180 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date conditional NADA NADA141544 04/29/2021 09/01/2024 KBROVET-CA1 500
potassium bromide tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:49427-398 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM BROMIDE (UNII: OSD78555ZM) (BROMIDE ION - UNII:952902IX06) POTASSIUM BROMIDE 500 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) 385.7 mg DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) 266 mg STEARIC ACID (UNII: 4ELV7Z65AP) 121 mg Product Characteristics Color brown (tan;with;brown;speckles) Score no score Shape ROUND Size 11mm Flavor VANILLA Imprint Code 500;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49427-398-48 60 in 1 PACKAGE 2 NDC:49427-398-50 180 in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date conditional NADA NADA141544 04/01/2023 Labeler - Pegasus Laboratories, Inc. (108454760) Registrant - Pegasus Laboratories, Inc. (108454760) Establishment Name Address ID/FEI Business Operations Pegasus Laboratories, Inc. 108454760 analysis, manufacture, label Establishment Name Address ID/FEI Business Operations Biospectra 042724830 api manufacture