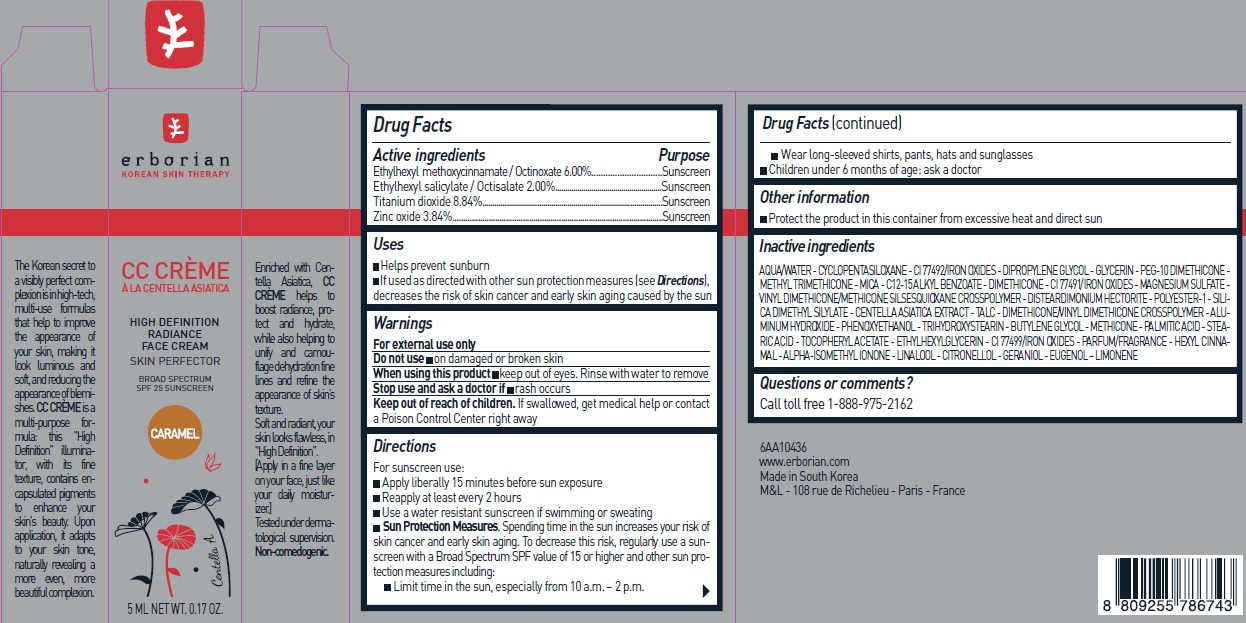
Label: ERBORIAN CC CREME HIGH DEFINITION SPF 25 CARAMEL- octinoxate, octisalate, titanium dioxide, zinc oxide cream
- NDC Code(s): 10345-913-05, 10345-913-45
- Packager: LABORATOIRES M&L
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
For sunscreen use:
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. –2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
- Children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
AQUA/WATER- CYCLOPENTASILOXANE - CI 77492/IRON OXIDES - DIPROPYLENE GLYCOL - GLYCERIN - PEG-10 DIMETHICONE - METHYL TRIMETHICONE - METHYL TRIMETHICONE - MICA - C12-15 ALKYL BENZOATE - DIMETHICONE - CI 77491/IRON OXIDES - MAGNESIUM SULFATE - VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER - DISTEARDIMONIUM HECTORITE - POLYESTER-1 - SILICA DIMETHYL SILYLATE - CENTELLA ASIATICA EXTRACT - TALC - DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER - ALUMINUM HYDROXIDE - PHENOXYETHANOL - TRIHYDROXYSTEARIN - BUTYLENE GLYCOL - METHICONE - PALMITIC ACID - STEARIC ACID - TOCOPHERYL ACETATE - ETHYLHEXYLGLYCERIN - CI 77499/ IRON OXIDES - PARFUM/FRAGRANCE - HEXYL CINNAMAL - ALPHA-ISOMETHYLIONONE-LINALOOL - CITRONELLOL - GERANIOL - EUGENOL - LIMONENE
- Questions or comments
- Package Labeling: 10345-913-05
- Package Labeling: 10345-913-45
-
INGREDIENTS AND APPEARANCE
ERBORIAN CC CREME HIGH DEFINITION SPF 25 CARAMEL
octinoxate, octisalate, titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10345-913 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 60 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 20 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 88.4 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 38.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) FERRIC OXIDE RED (UNII: 1K09F3G675) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONE (UNII: 92RU3N3Y1O) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYESTER-10 (UNII: 212N9O2MMZ) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CENTELLA ASIATICA (UNII: 7M867G6T1U) TALC (UNII: 7SEV7J4R1U) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PHENOXYETHANOL (UNII: HIE492ZZ3T) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) METHICONE (20 CST) (UNII: 6777U11MKT) PALMITIC ACID (UNII: 2V16EO95H1) STEARIC ACID (UNII: 4ELV7Z65AP) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) FERROSOFERRIC OXIDE (UNII: XM0M87F357) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LINALOOL, (+/-)- (UNII: D81QY6I88E) EUGENOL (UNII: 3T8H1794QW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10345-913-45 1 in 1 BOX 08/21/2018 1 45 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10345-913-05 1 in 1 BOX 04/16/2021 2 5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/21/2018 Labeler - LABORATOIRES M&L (262533623)