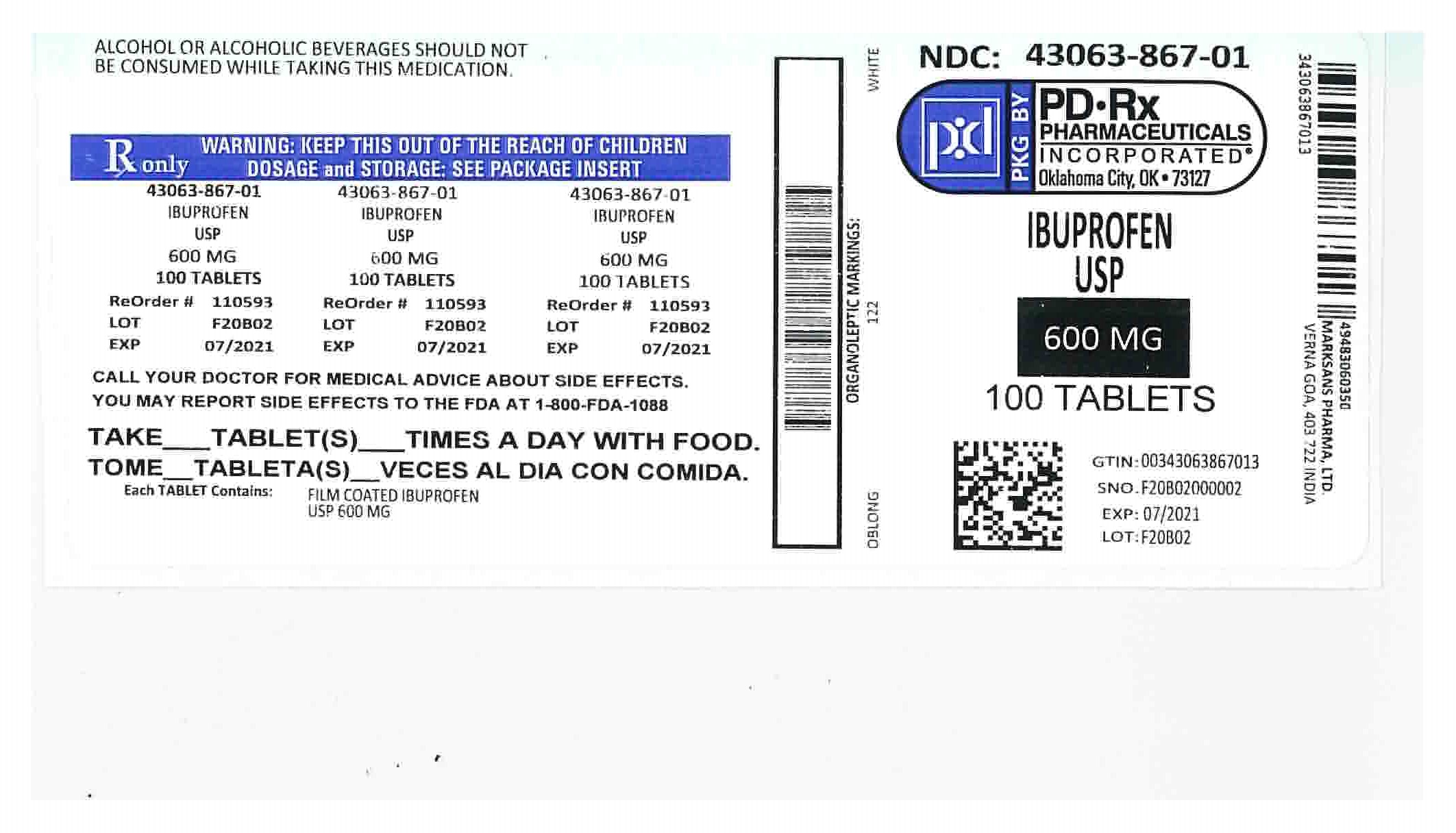
Label: IBUPROFEN tablet, film coated
- NDC Code(s): 43063-867-01, 43063-867-10, 43063-867-30, 43063-867-60
- Packager: PD-Rx Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 49483-603
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- ibuprofen tablets 400 mg - 600 mg- 800 mg medguide
- HOW SUPPLIED
- 600 mg
-
INGREDIENTS AND APPEARANCE
IBUPROFEN
ibuprofen tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43063-867(NDC:49483-603) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 600 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL (UNII: 532B59J990) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 122 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43063-867-10 10 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/05/2018 2 NDC:43063-867-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/05/2018 3 NDC:43063-867-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/11/2018 4 NDC:43063-867-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/30/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090796 12/30/2015 Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) Establishment Name Address ID/FEI Business Operations PD-Rx Pharmaceuticals, Inc. 156893695 repack(43063-867)