Label: OSMOLEX ER- amantadine tablet, extended release
-
NDC Code(s):
70482-075-07,
70482-075-14,
70482-075-30,
70482-076-07, view more70482-076-14, 70482-076-30
- Packager: Adamas Pharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 21, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
OSMOLEX ®ER - These highlights do not include all the information needed to use OSMOLEX ER safely and effectively. See full prescribing information for OSMOLEX ER.
OSMOLEX ®ER (amantadine) extended-release tablets, for oral use
Initial U.S. Approval: 1966INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- The initial dosage is 129 mg orally once daily in the morning (
2.1)
- The dosage may be increased in weekly intervals to a maximum daily dose of 322 mg once daily in the morning ( 2.1)
- Swallow tablets whole; do not chew, crush, or divide ( 2.2)
- Dosing frequency reduction and monitoring required for renal impairment (
2.3,
8.6)
DOSAGE FORMS AND STRENGTHS
Extended-release tablets containing 129 mg or 193 mg. (3)
CONTRAINDICATIONS
OSMOLEX ER is contraindicated in patients with end-stage renal disease ( 4)
WARNINGS AND PRECAUTIONS
- Falling Asleep During Activities of Daily Living and Somnolence: Advise patients prior to treatment; ordinarily discontinue if occurs ( 5.1)
- Suicidality and Depression: Monitor patients for depressed mood, depression, or suicidal ideation or behavior ( 5.2)
- Hallucinations/Psychotic Behavior: Patients with major psychotic disorder should ordinarily not be treated with OSMOLEX ER; observe patients for the occurrence of hallucinations throughout treatment, especially at initiation and after dose increases ( 5.3)
- Dizziness and Orthostatic Hypotension: Monitor patients for dizziness and orthostatic hypotension, especially after starting OSMOLEX ER or increasing the dose ( 5.4)
- Withdrawal-Emergent Hyperpyrexia and Confusion: Avoid sudden discontinuation ( 5.5)
- Impulse Control/Compulsive Behaviors: Ask patients about increased gambling urges, sexual urges, uncontrolled spending or other urges; consider dose reduction or discontinuation if occurs ( 5.6)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5%) are nausea, dizziness/ lightheadedness, and insomnia ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Adamas Pharma, LLC at 1-833-223-2627 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Anticholinergic Drugs: Increased risk of anticholinergic effects; the dose of anticholinergic drugs or OSMOLEX ER may require reduction ( 7.1)
- Drugs Affecting Urinary pH: Excretion increases with acidic urine; possible accumulation with urine change towards alkaline ( 7.2)
- Live Attenuated Influenza Vaccines: Not recommended during use ( 7.3)
- Alcohol: Concomitant use is not recommended; increased potential for CNS effects ( 7.4)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause fetal harm ( 8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2021
- The initial dosage is 129 mg orally once daily in the morning (
2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information
2.2 Administration Information
2.3 Dosing in Patients with Renal Impairment
2.4 Discontinuation, Dose Reduction, and Missed Dose
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Falling Asleep During Activities of Daily Living and Somnolence
5.2 Suicidality and Depression
5.3 Hallucination/Psychotic Behavior
5.4 Dizziness and Orthostatic Hypotension
5.5 Withdrawal-Emergent Hyperpyrexia and Confusion
5.6 Impulse Control/Compulsive Behaviors
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Other Anticholinergic Drugs
7.2 Drugs Affecting Urinary pH
7.3 Live Attenuated Influenza Vaccines
7.4 Alcohol
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information
The recommended initial dosage of OSMOLEX ER is 129 mg administered orally once daily in the morning. The dosage may be increased in weekly intervals to a maximum daily dose of 322 mg (administered as a 129 mg and 193 mg tablet), taken in the morning.
OSMOLEX ER is not interchangeable with other amantadine immediate- or extended-release products.
For patients unable to tolerate more than 100 mg per day of immediate-release amantadine, there is no equivalent dose or dosing regimen of OSMOLEX ER.
2.2 Administration Information
OSMOLEX ER should be swallowed whole. Do not chew, crush, or divide tablets. OSMOLEX ER can be administered without regard to food.
2.3 Dosing in Patients with Renal Impairment
There are no modifications for the recommended initial and maximum dosage in patients with renal impairment; however, modifications are recommended for the titration interval and frequency of dosing in patients with moderate and severe renal impairment (see Table 1).
Table 1: Recommended Titration and Frequency of OSMOLEX ER Dosing in Patients with Renal Impairment Renal Function/Estimated GFR (mL/min/1.73 m 2) * Minimum Titration Interval Frequency of Dosing Regimen Mild renal impairment (60 to 89) Increase every week One dose every 24 hours Moderate renal impairment (30 to 59) Increase every 3 weeks One dose every 48 hours Severe renal impairment (15 to 29) Increase every 4 weeks One dose every 96 hours End-Stage Renal Disease (below 15) Contraindicated * estimated by Modification of Diet in Renal Disease (MDRD) method Monitor patients with renal impairment for change in renal function, especially in those with severe renal impairment receiving the maximum daily dosage of 322 mg [see Use in Specific Populations (8.6)] .
2.4 Discontinuation, Dose Reduction, and Missed Dose
OSMOLEX ER should not be discontinued abruptly. The dose should be reduced gradually from higher doses to 129 mg daily for 1 to 2 weeks before discontinuing [see Warnings and Precautions (5.5)] .
If a dose of OSMOLEX ER is missed, the next dose should be taken as scheduled.
-
3 DOSAGE FORMS AND STRENGTHS
OSMOLEX ER is available as extended-release tablets for oral administration:
- Tablets containing 129 mg amantadine: Round, biconvex, white coated tablets, imprinted on one side with a black “VP” over “075”
- Tablets containing 193 mg amantadine: Round, biconvex, green coated tabled, imprinted on one side with a black “VP” over “076”
-
4 CONTRAINDICATIONS
OSMOLEX ER is contraindicated in patients with end-stage renal disease (i.e., creatinine clearance below 15 mL/min/1.73 m2) [see Clinical Pharmacology (12.3)] .
-
5 WARNINGS AND PRECAUTIONS
5.1 Falling Asleep During Activities of Daily Living and Somnolence
Patients treated with amantadine have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes has resulted in accidents. Patients may not perceive warning signs, such as excessive drowsiness, or they may report feeling alert immediately prior to the event.
Before initiating treatment with OSMOLEX ER, advise patients of the potential to develop drowsiness and specifically ask about factors that may increase the risk for somnolence with OSMOLEX ER, such as concomitant sedating medications, alcohol, or the presence of a sleep disorder. If a patient develops daytime sleepiness or episodes of falling asleep during activities that require full attention (e.g., driving a motor vehicle, conversations, eating), OSMOLEX ER should ordinarily be discontinued.
If a decision is made to continue OSMOLEX ER, advise patients not to drive and to avoid other potentially dangerous activities that might result in harm if they become somnolent. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living or daytime somnolence.
5.2 Suicidality and Depression
Suicide, suicide attempts, and suicidal ideation have been reported in patients with and without prior history of psychiatric illness while treated with amantadine. Amantadine can exacerbate psychiatric symptoms in patients with a history of psychiatric disorders or substance abuse.
Monitor patients for depression, including suicidal ideation or behavior. Prescribers should consider whether the benefits outweigh the risks of treatment with OSMOLEX ER in patients with a history of suicidality or depression.
5.3 Hallucination/Psychotic Behavior
Patients with a major psychotic disorder should ordinarily not be treated with OSMOLEX ER because of the risk of exacerbating psychosis. Treatment with amantadine or abrupt withdrawal can cause confusion, psychosis, personality changes, agitation, aggressive behavior, hallucinations, paranoia, other psychotic or paranoia reactions [see Warnings and Precautions (5.5)] .
Monitor patients for hallucinations throughout treatment but especially after initiation and after the dose of OSMOLEX ER is increased or decreased.
5.4 Dizziness and Orthostatic Hypotension
Dizziness and orthostatic hypotension can occur with OSMOLEX ER. Patients should be monitored for these adverse reactions, especially after starting OSMOLEX ER or increasing the dose. Concomitant use of alcohol when using OSMOLEX ER is not recommended [see Drug Interactions (7.4)] .
5.5 Withdrawal-Emergent Hyperpyrexia and Confusion
A symptom complex resembling neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in drugs that increase central dopaminergic tone.
Abrupt discontinuation of OSMOLEX ER may cause an increase in the symptoms of Parkinson’s disease or cause delirium, agitation, delusions, hallucinations, paranoid reaction, stupor, anxiety, depression, or slurred speech. It is recommended to avoid sudden discontinuation of OSMOLEX ER [see Dosage and Administration (2.4)] .
5.6 Impulse Control/Compulsive Behaviors
Patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone, including OSMOLEX ER. In some cases, these urges were reported to have stopped when the dose was reduced or the medication was stopped. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending, or other urges while being treated with OSMOLEX ER. Consider dose reduction or stopping the medication if a patient develops such urges while taking OSMOLEX ER.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Immediate-Release Amantadine
The adverse reactions listed in Table 2 were identified in clinical studies of immediate-release amantadine. The most common adverse reactions reported in ≥5% of patients at the recommended dosage of immediate-release amantadine were nausea, dizziness/lightheadedness, and insomnia.
Table 2: Incidence of Adverse Reactions from Pooled Studies of Immediate-Release Amantadine 5 to 10% 1 to 5% 0.1 to 1% Less than 0.1% Nausea Depression Congestive heart failure Convulsion Dizziness/ lightheadedness Anxiety and irritability Psychosis Leukopenia Insomnia Hallucinations Urinary retention Neutropenia Confusion Dyspnea Eczematoid dermatitis Anorexia Skin rash Oculogyric episodes Dry mouth Vomiting Suicidal attempt Constipation Weakness Suicide Ataxia Slurred speech Suicidal ideation Livedo reticularis Euphoria Peripheral edema Thinking abnormality Orthostatic hypotension Amnesia Headache Hyperkinesia Somnolence Hypertension Nervousness Decreased libido Dream abnormality Visual disturbance Agitation Punctate subepithelial
or other corneal opacityDry nose Corneal edema Diarrhea
Decreased visual acuity Fatigue Sensitivity to light Optic nerve palsy
-
7 DRUG INTERACTIONS
7.1 Other Anticholinergic Drugs
Products with anticholinergic properties may potentiate the anticholinergic-like side effects of amantadine. The dose of anticholinergic drugs or of OSMOLEX ER should be reduced if atropine-like effects appear when these drugs are used concurrently [see Warnings and Precautions (5.4)] .
7.2 Drugs Affecting Urinary pH
The pH of the urine has been reported to influence the excretion rate of amantadine. Urine pH is altered by diet, drugs (e.g., carbonic anhydrase inhibitors, sodium bicarbonate), and clinical state of the patient (e.g., renal tubular acidosis or severe infections of the urinary tract).
Since the excretion rate of amantadine increases rapidly when the urine is acidic, the administration of urine acidifying drugs may increase the elimination of the drug from the body. Alterations of urine pH towards the alkaline condition may lead to an accumulation of the drug with a possible increase in adverse reactions. Monitor for efficacy or adverse reactions under conditions that alter the urine pH to more acidic or alkaline, respectively.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with use of amantadine in pregnant women. Animal studies suggest a potential risk for fetal harm with amantadine. In mice and rats, adverse developmental effects (embryolethality, increased incidence of malformations, and reduced fetal body weight) were observed when amantadine was administered to pregnant animals at clinically relevant doses [see Data].
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk for major birth defects and miscarriage for the indicated populations is unknown.
Data
Animal Data
The effects of amantadine on development have not been tested in studies conducted in animals using currently recommended methodology; however, developmental toxicity studies of amantadine have been reported in the published literature.
In mice, oral administration of amantadine (0, 10, or 40 mg/kg/day) to pregnant animals during organogenesis (gestation days 7-12) resulted in embryolethality and reduced fetal body weight at the highest dose tested, which was associated with maternal toxicity. The no-effect dose for developmental toxicity in mice (10 mg/kg/day) is less than the maximum recommended human dose (MRHD) of 322 mg/day, based on body surface area (mg/m 2).
In rats, oral administration of amantadine (0, 40 or 120 mg/kg/day) to pregnant animals during organogenesis (gestation days 7-12) resulted in embryolethality and reduced fetal body weight at the highest dose. The no-effect dose for developmental toxicity in this study (40 mg/kg/day) is similar to the MRHD on a mg/m 2basis.
In another study in pregnant rats, oral administration of amantadine during organogenesis (gestation days 7-14) resulted in an increase in visceral and skeletal malformations at oral doses of 50 and 100 mg/kg/day. The no-effect dose for teratogenicity in this study (37 mg/kg/day) is similar to the MRHD on a mg/m 2basis.
Evaluation of parturition, lactation, and post-natal development in a limited number of litters from the mouse and rat studies described above revealed reductions in live litter size and pup weights at birth at 40 mg/kg/day in mice and 120 mg/kg/day in rats.
8.2 Lactation
Risk Summary
Amantadine is excreted in human milk, but amounts have not been quantified. There is no information on the risk to a breastfed infant, and there is insufficient information on the effect of amantadine on milk production in nursing mothers.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OSMOLEX ER and any potential adverse effects on the breastfed infant from OSMOLEX ER or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of OSMOLEX ER in pediatric patients have not been established.
8.5 Geriatric Use
No dose adjustment is recommended on the basis of age. OSMOLEX ER is known to be substantially excreted by the kidney, and the risk of adverse reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Dosage and Administration (2.3)] .
8.6 Renal Impairment
Because amantadine is mainly excreted in the urine, it accumulates in the plasma and in the body when renal function declines [see Clinical Pharmacology (12.3)] .
OSMOLEX ER is contraindicated for use in patients with end-stage renal disease (creatinine clearance below 15 mL/min/1.73 m 2).
For patients with moderate or severe renal impairment, a reduction in dosing frequency is required. Closely monitor these patients (creatinine clearance 15 to less than 60 mL/min/1.73 m 2) if prescribed the maximum daily dosage of 322 mg [see Dosage and Administration (2.3)] .
Also, closely monitor patients with any degree of renal impairment for adverse reactions and potential changes in renal function, which may necessitate further dosage reduction.
-
10 OVERDOSAGE
Deaths have been reported from overdose with amantadine. The lowest reported acute lethal dose was 1 gram of amantadine hydrochloride (equivalent to 0.8 g amantadine). Acute toxicity may be attributable to the anticholinergic effects of amantadine. Drug overdose has resulted in cardiac, respiratory, renal, or central nervous system toxicity. Pulmonary edema and respiratory distress (including adult respiratory distress syndrome, ARDS) have been reported with amantadine; renal dysfunction can occur, including increased BUN, decreased creatinine clearance, and renal insufficiency.
Central nervous system adverse reactions that have been reported with amantadine overdose include: agitation, aggressive behavior, hypertonia, hyperkinesia, ataxia, tremor disorientation, depersonalization, fear, delirium, psychotic reactions, lethargy, and coma. Seizures may be exacerbated in patients with prior history of seizure disorders. Hyperthermia has occurred with amantadine overdose.
For acute overdose, general supportive measures should be employed along with immediate gastric decontamination if appropriate. Give intravenous fluids if necessary. The excretion rate of amantadine increases with acidification of the urine, which may increase the elimination of the drug. Monitor patients for arrhythmias and hypotension. Electrocardiographic monitoring may be needed after ingestion because arrhythmias have been reported after overdose, including arrhythmias with fatal outcomes. Adrenergic agents, such as isoproterenol, in patients with an amantadine overdose has been reported to induce arrhythmias.
Monitor blood electrolytes, urine pH, and urinary output. Although hemodialysis does not efficiently remove amantadine, it may be useful in the treatment of amantadine toxicity in patients with renal failure.
-
11 DESCRIPTION
OSMOLEX ER contains amantadine in an extended-release tablet. The active ingredient in OSMOLEX ER is amantadine hydrochloride.
The chemical name for amantadine hydrochloride is tricyclo [3.3.1.1 3,7] decan-1-amine, hydrochloride or 1-adamantanamine hydrochloride, and it has the following structural formula:
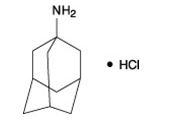
The molecular formula is C10H17N•HCl and the molecular weight is 187.71 (g/mol). Amantadine hydrochloride, USP is a stable white or nearly white crystalline powder, freely soluble in water and alcohol, and soluble in chloroform.
OSMOLEX ER tablets are for oral use. Each tablet contains 129 mg, or 193 mg amantadine (as 160 mg or 240 mg, amantadine hydrochloride, respectively) in an extended-release core and an outer immediate-release layer. Amantadine release from the extended-release core is controlled by an osmotic pump system. Osmotic pump systems consist of a drug core contained within a semipermeable polymer membrane that is permeable to water molecules, but not to the drug, with a laser drilled orifice for drug delivery. Amantadine release is driven by the existence of an osmotic gradient between the contents of the drug core and the fluid in the gastrointestinal tract. Since the osmotic gradient remains constant, drug delivery remains essentially constant after the immediate-release layer dissolves. The biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the stool as a tablet shell.
Inactive ingredients: cellulose acetate, colloidal silicon dioxide, copovidone, D&C Yellow No. 10, FD&C Yellow No. 6, ferrosoferric oxide, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, propylene glycol, sodium chloride, and titanium dioxide. OSMOLEX ER 129 mg tablets also contain lactose monohydrate and triacetin. OSMOLEX ER 193 mg tablets also contain FD&C Blue No. 2 and FD&C Red No. 40.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism by which amantadine exerts efficacy in the treatment of Parkinson’s disease and drug-induced extrapyramidal reactions is unknown. Amantadine is a weak uncompetitive antagonist of the NMDA receptor; however, it exhibits anticholinergic-like side effects such as dry mouth, urinary retention, and constipation in humans. Amantadine may have direct and indirect effects on dopamine neurons; it exerts dopaminergic-like side effects such as hallucinations and dizziness in humans.
12.2 Pharmacodynamics
The effect of amantadine on QT prolongation was not studied in a dedicated thorough QT study.
Alcohol consumption may increase the potential for CNS effects, such as somnolence, dizziness, confusion, lightheadedness, and orthostatic hypotension [see Drug Interactions (7.4)] .
12.3 Pharmacokinetics
OSMOLEX ER tablet consists of an immediate-release outer layer and an extended-release core. The pharmacokinetics of amantadine from OSMOLEX ER is dose proportional across the dose range of 129 mg to 322 mg.
Absorption
Following oral administration of OSMOLEX ER, peak concentration of amantadine was observed in a median time of 7.5 hours (range 5.5 to 12 hours). After a single oral administration of the 129 mg dose, the mean (CV%) Cmax and AUC were 328 ng/ml (18%) and 8263 ng.h/ml (18%) respectively. Cmax and AUC with other dose levels of OSMOLEX ER increase proportionally.
Effect of Food
Food does not affect the rate or the extent of absorption of OSMOLEX ER.
Distribution
Amantadine is 67% bound to plasma proteins over a concentration range of 0.1 to 2.0 μg/mL. The volume of distribution after intravenous administration is 3-8 L/kg, suggesting potential extravascular distribution.
Elimination
Amantadine is mainly eliminated renally, and approximately 85% of the administered dose is excreted unchanged in urine. After oral administration of a single 129 mg OSMOLEX ER tablet, the apparent oral clearance was approximately11 L/h. The half-life was approximately 16 hours.
Metabolism
Metabolism accounts for only 5-15% of the total clearance for amantadine. Eight metabolites of amantadine have been identified in human urine. One metabolite, an N-acetylated compound, was quantified in human urine and accounted for 0-15% of the administered dose in multiple studies. The contribution of this metabolite to efficacy or toxicity is not known.
Excretion
Amantadine is primarily excreted by glomerular filtration and tubular secretion. The pH of the urine has been reported to influence the excretion rate of amantadine [see Drug Interactions (7.2)] .
Specific Populations
Geriatric Patients
Amantadine is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function [see Use in Specific Populations (8.5)] .
Patients with Renal Impairment
The renal clearance of amantadine is significantly lower in patients with moderate or severe renal impairment, compared to healthy subjects with normal renal function. Therefore, a reduction in dosing frequency is required for patients with moderate or severe renal impairment [see Dosage and Administration (2.3)] . OSMOLEX ER is contraindicated in patients with end-stage renal disease (i.e., creatinine clearance below 15 mL/min/1.73 m2) [see Contraindications (4)] .
Male and Female Patients
In a study of young healthy subjects (n=20), mean renal clearance of amantadine, normalized for body mass index, was 1.5 fold higher in males compared to females. No dose adjustment by gender is warranted.
Drug Interaction Studies
Coadministration of quinine or quinidine with amantadine was shown to reduce the renal clearance of amantadine by about 30%. The clinical significance of this effect is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
Carcinogenesis
Animal studies designed to evaluate the carcinogenic potential of amantadine have not been conducted.
Mutagenesis
Amantadine was negative for genotoxicity in in vitro (Ames and mammalian cell [Chinese Hamster ovary and human peripheral blood lymphocytes]) assays in the presence or absence of metabolic activation and in an in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
The effects of amantadine on fertility have not been adequately tested in a study in animals conducted according to current standards. In a reproduction study reported in the literature, oral administration of amantadine to male and female rats at a dose of 32 mg/kg/day resulted in impaired fertility. The no-effect dose for adverse effects on fertility (10 mg/kg/day) is less than the maximum recommended human dose of 322 mg/day on a mg/m 2basis.
- 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
OSMOLEX ER is available as extended-release tablets in the following configurations:
129 mg tablets:
Round, biconvex, white coated tablets, imprinted on one side with a black “VP” over “075”
- Bottles of 30: NDC 70482-075-30
193 mg tablets:
Round, biconvex, green coated tabled, imprinted on one side with a black “VP” over “076”
- Bottles of 30: NDC 70482-076-30
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration
Instruct patients and caregivers that OSMOLEX ER tablets should be swallowed whole and can be administered with or without food. Advise patients to speak to their healthcare provider before discontinuation of OSMOLEX ER [see Dosage and Administration ( 2.2, 2.4)] . The OSMOLEX ER tablet has a nonabsorbable shell designed to release the drug at a controlled rate. The tablet shell is eliminated from the body; patients should not be concerned if they occasionally notice in their stool something that looks like a tablet [see Description (11)] .
Falling Asleep During Activities of Daily Living
Advise patients that sleepiness and fatigue can occur with OSMOLEX ER, and patients treated with amantadine have reported falling asleep while engaged in activities of daily living. These adverse reactions may affect some patients’ ability to drive and operate machinery safely [see Warnings and Precautions (5.1)] .
Suicidality and Depression
Instruct patients, family members, and caregivers to notify their healthcare provider if depressed mood, depression, changes in behavior or thinking, or suicidal ideation or behavior develop during treatment [see Warnings and Precautions (5.2)] .
Hallucinations/Psychotic Behavior
Inform patients and caregivers that hallucinations and paranoia can occur while taking OSMOLEX ER. Tell patients to report unreal visions, sounds, or sensations or other psychotic behavior to their healthcare provider promptly should they develop [see Warnings and Precautions (5.3)] .
Dizziness and Orthostatic Hypotension
Dizziness and orthostatic hypotension can occur with administration of OSMOLEX ER. Caution patients against standing rapidly after sitting or lying down, especially if they have been doing so for prolonged periods and especially at the initiation of treatment with OSMOLEX ER [see Warnings and Precautions (5.4)] .
Withdrawal-Emergent Hyperpyrexia and Confusion
Advise patients to contact their healthcare provider before stopping OSMOLEX ER. Tell patients to inform their healthcare provider if they develop withdrawal symptoms such as fever, confusion, or severe muscle stiffness [see Warnings and Precautions (5.5)] .
Impulse Control/Compulsive Disorders
Inform patients of the potential for experiencing intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and other intense urges and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone, such as OSMOLEX ER [see Warnings and Precautions (5.6)] .
Drug Interactions
Certain medications can cause an interaction with OSMOLEX ER. Advise patients and/or caregivers to inform their healthcare provider of all the medicines the patient is taking, including over-the-counter medicines, dietary supplements, and herbal products. Inform patients that live influenza vaccines and consumption of alcohol are not recommended during treatment with OSMOLEX ER [see Drug Interactions ( 7.1, 7.2, 7.3, 7.4)] .
-
PATIENT MEDICATION INFORMATION
PATIENT INFORMATION
OSMOLEX ER (Oz-mole-x ee-are)
(amantadine) extended release tabletsWhat is OSMOLEX ER?
OSMOLEX ER is a prescription medicine used to treat:- Parkinson’s disease
- Drug-induced extrapyramidal reactions in adult patients
It is not known if OSMOLEX ER is safe and effective in children.
Do not take OSMOLEX ER if youhave severe kidney problems. Before taking OSMOLEX ER, tell your doctor about all of your medical conditions, including if you:
- have kidney problems.
- have daytime sleepiness from a sleep disorder, have unexpected or unpredictable sleepiness or periods of sleep, take a medicine to help you sleep, or take any medicine that makes you drowsy.
- have mental health problems, such as suicidal thoughts, depression, or hallucinations.
- have unusual urges including gambling, increased sex drive, compulsive eating, or compulsive shopping.
- drink alcohol. Drinking alcohol may increase your chances of becoming drowsy, sleepy, confused, dizzy, light headed, or faint while taking OSMOLEX ER.
- are pregnant or plan to become pregnant. OSMOLEX ER may harm your unborn baby.
- are breastfeeding or plan to breastfeed. OSMOLEX ER can pass into your breast milk. Talk to your doctor about the best way to feed your baby if you take OSMOLEX ER.
Tell your doctor about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and dietary and herbal supplements. OSMOLEX ER may affect the way certain other medicines work, and certain other medicines can affect how OSMOLEX ER works.
Especially tell your doctor if you:
- take sodium bicarbonate or a medicine that contains sodium
bicarbonate. - are planning to receive a flu (influenza) vaccine. You may receive the flu vaccine shot but should notget a live flu vaccine (nasal spray) while taking OSMOLEX ER.
How should I take OSMOLEX ER?
- Take OSMOLEX ER exactly as your doctor tells you to.
- Start OSMOLEX ER with 1 tablet in the morning. Your doctor may change your dose if needed.
- Do notstop taking OSMOLEX ER or change your dose before talking with your doctor. Tell your doctor if you have symptoms of withdrawal such as fever, confusion, or severe muscle stiffness.
- OSMOLEX ER may be taken with food or without food.
- Swallow OSMOLEX ER tablets whole. Do not chew, crush, or divide OSMOLEX ER tablets.
- If you miss a dose of OSMOLEX ER, do not make up the missed dose. Take your usual dose of OSMOLEX ER on the next day in the morning.
- If you take too much OSMOLEX ER, call your doctor or go to the nearest hospital emergency room right away.
- OSMOLEX ER should not be used instead of other immediate-release amantadine medicines or other extended-release amantadine medicines.
- The OSMOLEX ER tablet shell does not dissolve completely even after all the drug has been released. The tablet shell may be seen in your stool.
What should I avoid while taking OSMOLEX ER?
- Do notdrive, operate machinery, or do other dangerous activities until you know how OSMOLEX ER affects you. If you have become sleepy during the day or have fallen asleep while doing normal daily activities during treatment with OSMOLEX ER, do notdrive, operate machinery, or do other dangerous activities that could cause harm if you become sleepy.
- You should notdrink alcohol during treatment with OSMOLEX ER. Drinking alcohol can increase your chances of getting serious side effects.
What are the possible side effects of OSMOLEX ER?
OSMOLEX ER may cause serious side effects, including:
- falling asleep during normal activities.You may fall asleep while doing normal activities such as driving a car, talking, or eating while taking OSMOLEX ER. You may fall asleep without being drowsy or without warning. This may result in having accidents. Your chances of falling asleep while doing normal activities while taking OSMOLEX ER are greater if you take other medicines that cause drowsiness or drink alcohol. Tell your doctor right away if this happens.
- suicidal thoughts or actions and depression.Some people taking amantadine who have a history of mental illness and some people who do not have a history of mental illness, have had depression, suicidal thoughts, attempted suicide, and have committed suicide. Amantadine can make psychiatric symptoms in people with a history of mental health problems or a history of substance abuse worse. Tell your doctor right away if you have changes in your mood, behaviors, or thoughts, including thoughts about hurting yourself or ending your life.
-
seeing, hearing, or feeling things that are not real (hallucinations) and behaviors of being out of touch with reality (psychotic behaviors).Taking amantadine or stopping amantadine suddenly can cause confusion, psychosis, changes in personality, agitation, aggressiveness, hallucinations, and not trusting and being suspicious of others for no reason (paranoia). Tell your doctor right away if you have any of these changes in your behavior.
- feeling dizzy, faint or light headed, especially when you stand up (orthostatic hypotension).Dizziness, light headedness, or fainting can happen with OSMOLEX ER when getting up too quickly from a sitting or lying position, especially after a long period of time, and especially when first starting OSMOLEX ER or if your dose has been increased. Stand up slowly when moving from a sitting or lying position. Tell your doctor if you become dizzy, light headed or faint when standing up.
- unusual urges.Some people taking OSMOLEX ER get urges to behave in a way unusual for them. Examples of this are strong urges to gamble, increased sexual urges, strong urges to spend money, binge eating and the inability to control these urges. If you notice or your family notices that you are developing any unusual behaviors, talk to your doctor.
The most common side effects of OSMOLEX ER includenausea, dizziness, lightheadedness, and trouble sleeping (insomnia).
These are not all the possible side effects of OSMOLEX ER. Call your doctor for medical advice about side effects. You may report side effects to Adamas Pharma, LLC at 1-833-223-2627 or FDA at 1-800-FDA-1088.
How should I store OSMOLEX ER?
- Store OSMOLEX ER at room temperature between 68°F to 77°F (20°C to 25°C).
Keep OSMOLEX ER and all medicines out of the reach of children.
General information about the safe and effective use of OSMOLEX ER.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use OSMOLEX ER for a condition for which it was not prescribed. Do not give OSMOLEX ER to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about OSMOLEX ER that is written for health professionals.
What are the ingredients in OSMOLEX ER?
Active ingredient:amantadine hydrochloride
Inactive ingredients:cellulose acetate, colloidal silicon dioxide, copovidone, D&C Yellow No. 10, FD&C Yellow No. 6, ferrosoferric oxide, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, propylene glycol, sodium chloride, and titanium dioxide. OSMOLEX ER 129 mg tablets also contain lactose monohydrate and triacetin. OSMOLEX ER 193 mg tablets also contain FD&C Blue No. 2 and FD&C Red No. 40.
Manufactured for: Adamas Pharma, LLC. Emeryville, CA 94608
For more information, go to www.OSMOLEX-ER.com or call 1-833-223-2627.This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 03/2021 - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OSMOLEX ER
amantadine tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70482-075 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMANTADINE HYDROCHLORIDE (UNII: M6Q1EO9TD0) (AMANTADINE - UNII:BF4C9Z1J53) AMANTADINE 129 mg Inactive Ingredients Ingredient Name Strength CELLULOSE ACETATE (UNII: 3J2P07GVB6) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE K25-31 (UNII: D9C330MD8B) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white Score no score Shape ROUND Size 8mm Flavor Imprint Code VP075 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70482-075-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2018 05/31/2025 2 NDC:70482-075-07 2 in 1 CARTON 06/01/2018 05/31/2025 2 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:70482-075-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2018 05/31/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209410 06/01/2018 05/31/2025 OSMOLEX ER
amantadine tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70482-076 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMANTADINE HYDROCHLORIDE (UNII: M6Q1EO9TD0) (AMANTADINE - UNII:BF4C9Z1J53) AMANTADINE 193 mg Inactive Ingredients Ingredient Name Strength CELLULOSE ACETATE (UNII: 3J2P07GVB6) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) COPOVIDONE K25-31 (UNII: D9C330MD8B) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FERROSOFERRIC OXIDE (UNII: XM0M87F357) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM CHLORIDE (UNII: 451W47IQ8X) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color green Score no score Shape ROUND Size 10mm Flavor Imprint Code VP076 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70482-076-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2018 06/30/2024 2 NDC:70482-076-07 2 in 1 CARTON 06/01/2018 06/30/2024 2 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:70482-076-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2018 06/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209410 06/01/2018 06/30/2024 Labeler - Adamas Pharma, LLC (192255110) Establishment Name Address ID/FEI Business Operations Osmotica Pharmaceutical US LLC 080299818 label(70482-076, 70482-075) , manufacture(70482-075, 70482-076) , pack(70482-075, 70482-076)








