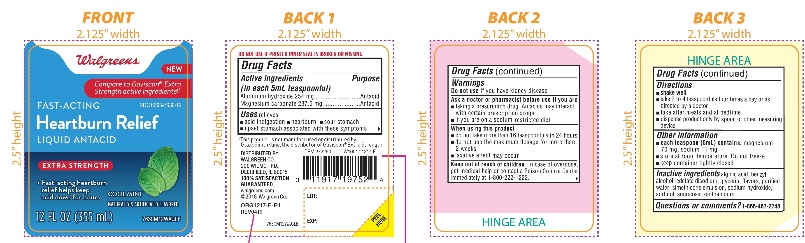
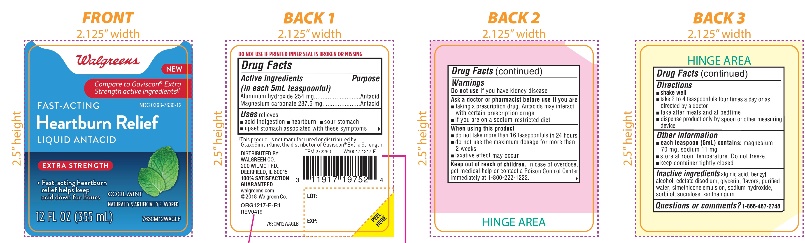
Label: WALGREENS FAST ACTING HEARTBURN RELIEF EXTRA STRENGTH- aluminum hydroxide and magnesium carbonate liquid
- NDC Code(s): 0363-7630-12
- Packager: WALGREEN CO.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 5mL teaspoonful)
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you are
- •
- taking a prescription drug. Antacids may interact with certain prescription drugs.
- •
- if you are on a sodium-restricted diet
- Directions
- Other information
- Inactive Ingredients
- Questions or comments?
-
Principal Display Panel
Walgreens
Compare to Gaviscon® Extra Strength active ingredients††
FAST-ACTING
HeartburnRelief
LIQUID ANTACID
Extra strength
- •
- Fast- acting heartburn relief helps keep acid down for hours
Cool Mint Flavor
Naturally and Artificially Flavored
12 FL OZ (355 ml)
*This product is not manufactured or distributed by GlaxoSmithKline, the distributor of Gaviscon® Extra Strength.
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
100% SATISFACTION GUARANTEED
Walgreens.com ©2018 Walgreen Co.
-
INGREDIENTS AND APPEARANCE
WALGREENS FAST ACTING HEARTBURN RELIEF EXTRA STRENGTH
aluminum hydroxide and magnesium carbonate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-7630 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 254 mg in 5 mL MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 237.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ALGINIC ACID (UNII: 8C3Z4148WZ) BENZYL ALCOHOL (UNII: LKG8494WBH) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) DIMETHICONE, UNSPECIFIED (UNII: 92RU3N3Y1O) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor MINT (cool mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-7630-12 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 01/15/2018 Labeler - WALGREEN CO. (008965063)