Label: NITROFURANTOIN ORAL SUSPENSION suspension
- NDC Code(s): 0121-1996-10, 0121-1996-95
- Packager: PAI Holdings, LLC dba PAI Pharma
- This is a repackaged label.
- Source NDC Code(s): 70408-239
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
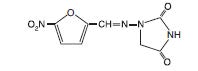
DESCRIPTIONNitrofurantoin, a synthetic chemical, is a stable, yellow, crystalline compound. Nitrofurantoin Oral Suspension, USP is an antibacterial agent for specific urinary tract ...
-
CLINICAL PHARMACOLOGYOrally administered Nitrofurantoin Oral Suspension, USP is readily absorbed and rapidly excreted in urine. Blood concentrations at therapeutic dosage are usually low. It is highly soluble in ...
-
INDICATIONS AND USAGE
Nitrofurantoin Oral Suspension, USP is specifically indicated for the treatment of urinary tract infections when due to susceptible strains of Escherichia coli, enterococci, Staphylococcus aureus ...
-
CONTRAINDICATIONS:
Anuria, oliguria, or significant impairment of renal function (creatinine clearance under 60 mL per minute or clinically significant elevated serum creatinine) are contraindications. Treatment of ...
-
WARNINGS:
Pulmonary reactions: ACUTE, SUBACUTE, OR CHRONIC PULMONARY REACTIONS - HAVE BEEN OBSERVED IN PATIENTS TREATED WITH NITROFURANTOIN. IF THESE REACTIONS OCCUR, NITROFURANTOIN ORAL SUSPENSION, USP ...
-
PRECAUTIONSInformation for Patients: Patients should be advised to take Nitrofurantoin Oral Suspension, USP with food to further enhance tolerance and improve drug absorption. Patients should be ...
-
ADVERSE REACTIONSRespiratory: CHRONIC, SUBACUTE, OR ACUTE PULMONARY HYPERSENSITIVITY REACTIONS MAY OCCUR. CHRONIC PULMONARY REACTIONS MAY OCCUR GENERALLY IN PATIENTS WHO HAVE RECEIVED CONTINUOUS TREATMENT FOR ...
-
OVERDOSAGEOccasional incidents of acute overdosage of Nitrofurantoin Oral Suspension, USP have not resulted in any specific symptoms other than vomiting. Induction of emesis is recommended. There is no ...
-
DOSAGE AND ADMINISTRATIONNitrofurantoin Oral Suspension, USP should be given with food to improve drug absorption and, in some patients, tolerance. Adults: 50 to 100 mg four times a day — the lower dosage level is ...
-
HOW SUPPLIEDNitrofurantoin Oral Suspension, USP is a lemon yellow liquid with a fruity scent available in: NDC 0121-1996-10: 10 mL unit dose cup. Case contains 10 unit-dose cups of 10 mL (NDC 0121-1996-95) ...
-
PRINCIPAL DISPLAY PANELNDC 0121-1996-05 - Nitrofurantoin Oral Suspension, USP - 50 mg/10 mL - FOR ORAL USE ONLY - A0998100124
-
INGREDIENTS AND APPEARANCEProduct Information