Label: FLUMIST- influenza vaccine live intranasal spray
- NDC Code(s): 66019-311-00, 66019-311-10
- Packager: MedImmune, LLC
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated August 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FLUMIST® safely and effectively. See full prescribing information for FLUMIST® FluMist® (Influenza Vaccine Live, Intranasal) Nasal ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEFluMist® is a vaccine indicated for active immunization for the prevention of influenza disease caused by influenza virus subtypes A and type B contained in the vaccine [see Description ...
-
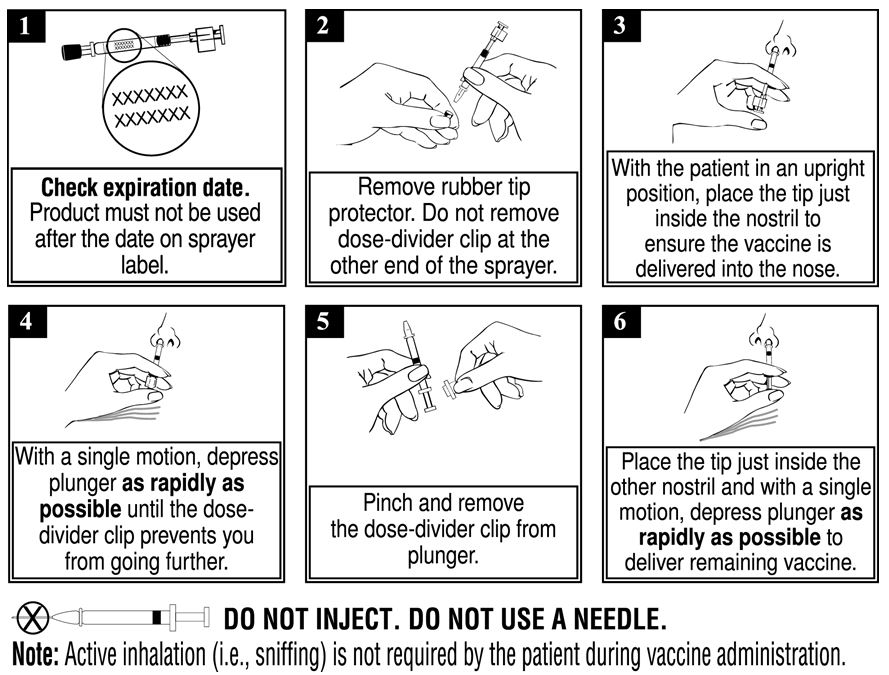
2 DOSAGE AND ADMINISTRATIONFor intranasal use. 2.1 Dosing Information - Administer FluMist according to the following schedule: * 1 or 2 doses depends on vaccination history as per Advisory Committee on ...
-
3 DOSAGE FORMS AND STRENGTHSFluMist is a nasal spray. A single dose is 0.2 mL.
-
4 CONTRAINDICATIONS 4.1 Severe Allergic Reactions - Do not administer FluMist to persons who have had a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine [see Description (11)] including ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risks of Hospitalization and Wheezing in Children Younger than 24 Months of Age - In clinical trials, risks of hospitalization and wheezing were increased in children younger than 2 years of ...
-
6 ADVERSE REACTIONSThe most common solicited adverse reactions (≥10% in vaccine recipients and at least 5% greater than in placebo recipients) reported after FluMist were runny nose or nasal congestion (ages 2 years ...
-
7 DRUG INTERACTIONS7.1 Aspirin Therapy - Do not administer FluMist to children and adolescents through 17 years of age who are receiving aspirin therapy or aspirin-containing therapy because of the association of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - FluMist is not absorbed systemically following intranasal administration and maternal use is not expected to result in fetal exposure to the drug. 8.2 ...
-
11 DESCRIPTIONFluMist (Influenza Vaccine Live, Intranasal) is a nasal spray. FluMist contains three vaccine virus strains: an A/H1N1 strain, an A/H3N2 strain and a B strain from the B/Victoria lineage. The ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Immune mechanisms conferring protection against influenza following receipt of FluMist vaccine are not fully understood; serum antibodies, mucosal antibodies, and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - FluMist has not been evaluated for its carcinogenic or mutagenic potential.
-
14 CLINICAL STUDIES14.1 Efficacy Studies of FluMist in Children - A multi-national, randomized, double-blind, active-controlled trial (MI-CP111) was performed to assess the efficacy of FluMist compared to an ...
-
15 REFERENCES1. Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barré syndrome and the 1992 – 1993 and 1993 – 1994 influenza vaccines. N Engl J Med 1998;339(25):1797-802.
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - FluMist is supplied in a package of 10 pre-filled, single-dose (0.2 mL) intranasal sprayers. The single-use intranasal sprayer is not made with natural rubber latex. Carton ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the vaccine recipient or caregiver to read the FDA-approved patient labeling (Information for Patients and Their Caregivers). Inform vaccine recipients or their parents/guardians of the ...
-
Information for Patients and Their CaregiversFluMist® (pronounced FLEW-mĭst) (Influenza Vaccine Live, Intranasal) Please read this Patient Information carefully before you or your child is vaccinated with FluMist. This is a summary of ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL 2024-2025 FORMULA - Influenza Vaccine Live, Intranasal - FluMist® STORE REFRIGERATED - at 2º-8ºC (35º-46ºF) RX only - For 2-49 years of age - PROTECT FROM LIGHT - FOR INTRANASAL USE - Contents: 10 pre-filled ...
-
INGREDIENTS AND APPEARANCEProduct Information