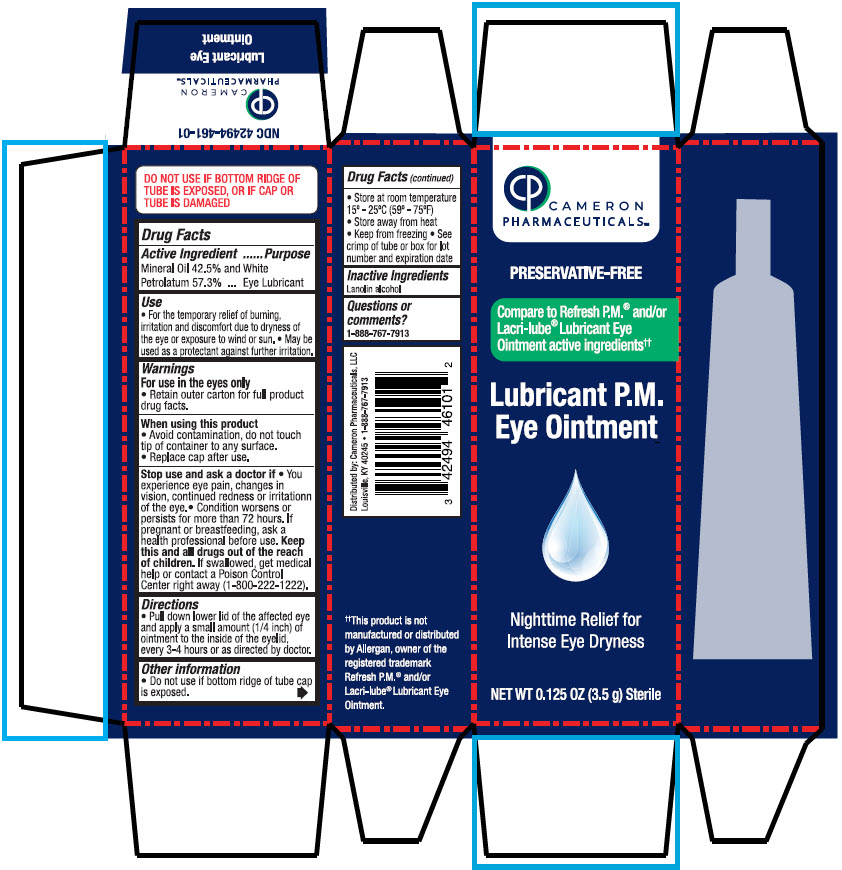
Label: LUBRICANT PM EYE- mineral oil and petrolatum solution/ drops
- NDC Code(s): 42494-461-01
- Packager: Cameron Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
-
Warnings
For use in the eyes only
- Retain outer carton for full product drug facts.
When using this product
- Avoid contamination, do not touch tip of container to any surface.
- Replace cap after use.
- Directions
- Other information
- Inactive Ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 3.5 g Bottle Carton
-
INGREDIENTS AND APPEARANCE
LUBRICANT PM EYE
mineral oil and petrolatum solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42494-461 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINERAL oil (UNII: T5L8T28FGP) (MINERAL oil - UNII:T5L8T28FGP) MINERAL oil 425 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 568 mg in 1 g Inactive Ingredients Ingredient Name Strength LANOLIN ALCOHOLS (UNII: 884C3FA9HE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42494-461-01 3.5 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 11/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M018 11/11/2024 Labeler - Cameron Pharmaceuticals (078371442)