Label: PRUCALOPRIDE tablet
- NDC Code(s): 70954-596-10, 70954-597-10
- Packager: ANI Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PRUCALOPRIDE TABLETS safely and effectively. See full prescribing information for PRUCALOPRIDE TABLETS. PRUCALOPRIDE tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS & USAGEPrucalopride tablet is indicated for the treatment of chronic idiopathic constipation (CIC) in adults.
-
2 DOSAGE & ADMINISTRATIONPrucalopride tablets can be taken with or without food. The recommended dosage by patient population is shown in Table 1. Table 1: Recommended Dosage Regimen and Dosage Adjustments by ...
-
3 DOSAGE FORMS & STRENGTHSPrucalopride Tablets: 1 mg prucalopride: White to off-white, round, biconvex film-coated tablets debossed with "N 596" on one side and plain on the other side, free from physical defects. 2 mg ...
-
4 CONTRAINDICATIONSPrucalopride tablets are contraindicated in patients with: A history of hypersensitivity to prucalopride. Reactions including dyspnea, rash, pruritus, urticaria, and facial edema have been ...
-
5 WARNINGS AND PRECAUTIONS5.1 Suicidal Ideation and Behavior - In clinical trials, suicides, suicide attempts, and suicidal ideation have been reported. Postmarketing cases of suicidal ideation and behavior as well as ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from case reports with prucalopride use in pregnant women are insufficient to identify any drug-associated risks of miscarriage, major birth defects ...
-
10 OVERDOSAGEAn overdose may result in appearance of symptoms from an exaggeration of the known pharmacodynamic effects of prucalopride and includes headache, nausea, and diarrhea. Specific treatment is not ...
-
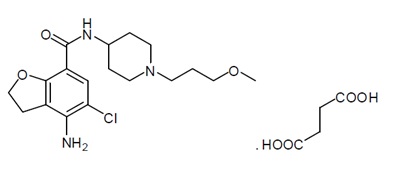
11 DESCRIPTIONPrucalopride tablets for oral use contain prucalopride succinate, a dihydrobenzofurancarboxamide that is a serotonin type 4 (5-HT4) receptor agonist. The IUPAC name is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Prucalopride, a selective serotonin type 4 (5-HT4) receptor agonist, is a gastrointestinal (GI) prokinetic agent that stimulates colonic peristalsis (high-amplitude ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility - Carcinogenesis - In a 2-year carcinogenicity study in mice, prucalopride was given by daily oral gavage at doses of 10, 20, and 80 ...
-
14 CLINICAL STUDIESThe efficacy of prucalopride tablets for the treatment of CIC was evaluated in six double-blind, placebo-controlled, randomized, multicenter clinical trials in 2484 adult patients (Studies 1 to 6 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPrucalopride Tablets containing 1 mg prucalopride are white to off-white, round, biconvex film-coated tablets debossed with "N 596" on one side and plain on the other side, free from physical ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information) Suicidal Ideation and Behavior: Inform patients, their caregivers, and family members that suicidal ideation ...
-
PATIENT INFORMATIONPATIENT INFORMATION - Prucalopride - (proo-kal-oh-pride) tablets, for oral use - What is prucalopride tablet? Prucalopride tablet is a prescription medicine used in adults to treat a type ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELPrucalopride Tablets, 1 mg - NDC 70954-596-10 - 30 Tablets - Prucalopride Tablets, 2 mg - NDC 70954-597-10 - 30 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information