Label: LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE tablet, film coated
- NDC Code(s): 43547-423-03, 43547-423-09, 43547-423-11, 43547-424-03, view more
- Packager: Solco Healthcare US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LOSARTAN POSTASSIUM AND HYDROCHLOROTHIAZIDETABLETS safely and effectively. See full prescribing information for LOSARTAN POTASSIUM ...
-
Table of ContentsTable of Contents
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE 1.1 Hypertension Losartan potassium and hydrochlorothiazide tablets are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Hypertension The usual starting dose of losartan potassium and hydrochlorothiazide tablets is 50/12.5 (losartan 50 mg/hydrochlorothiazide 12.5 mg) once daily. The dosage can be increased ...
-
3 DOSAGE FORMS AND STRENGTHS • Losartan potassium and hydrochlorothiazide tablets, USP 50/12.5 mg are yellow, capsule-shaped, film-coated tablets, debossed with “HH” on one side and “211” on the other side. • Losartan ...
-
4 CONTRAINDICATIONS Losartan potassium and hydrochlorothiazide tablets are contraindicated: • In patients who are hypersensitive to any component of this product. • In patients with anuria - • For coadministration ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Fetal Toxicity Losartan potassium and hydrochlorothiazide tablets can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during ...
-
6 ADVERSE REACTIONS 6.1 Clinical Trials Experience Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS 7.1 Agents Increasing Serum Potassium Coadministration of losartan with other drugs that raise serum potassium levels may result in hyperkalemia. Monitor serum potassium in such ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy Risk Summary - Losartan potassium and hydrochlorothiazide tablets can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin ...
-
10 OVERDOSAGE Losartan Potassium - Significant lethality was observed in mice and rats after oral administration of 1,000 mg/kg and 2,000 mg/kg, respectively, about 44 and 170 times the maximum recommended ...
-
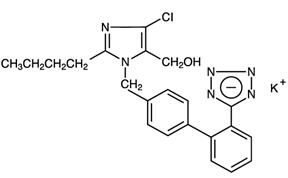
11 DESCRIPTION Losartan potassium and hydrochlorothiazide tablets, 50/12.5 mg, losartan potassium and hydrochlorothiazide tablets, 100/12.5 mg and losartan potassium and hydrochlorothiazide tablets, 100/25 mg ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action Losartan Potassium - Angiotensin II [formed from angiotensin I in a reaction catalyzed by angiotensin converting enzyme (ACE, kininase II)], is a potent ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility Losartan Potassium-Hydrochlorothiazide - No carcinogenicity studies have been conducted with the losartan ...
-
14 CLINICAL STUDIES 14.1 Losartan Monotherapy Reduction in the Risk of Stroke: The LIFE study was a multinational, double-blind study comparing losartan and atenolol in 9,193 hypertensive patients with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Losartan potassium and hydrochlorothiazide tablets, USP are supplied as a film-coated tablet. Losartan/ Hydrochlorothiazide - Color - Shape - Engraving - NDC ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Pregnancy: Advise female patients of childbearing age about the consequences of exposure to losartan potassium ...
-
FDA-approved patient labelingDispense with Patient Information available at: www.solcohealthcare.com/druglabeling/losartan-hctz-tablets.pdf - Patient Information - Losartan Potassium and Hydrochlorothiazide Tablets, USP ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL – 50/12.5 mg – 90 tablets Label-Existing Manufacturing Site Zhejiang Huahai Xunqiao Container Label – 50/12.5 mg – 90 tablets - Rx only - NDC 43547-423-09 - Losartan Potassium and Hydrochlorothiazide Tablets, USP - Pharmacist: Dispense the Patient Information provided separately ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL – 100/12.5 mg – 90 tablets Label-Existing Manufacturing Site Zhejiang Huahai Xunqiao Container Label – 100/12.5 mg – 90 tablets - Rx only - NDC 43547-425-09 - Losartan Potassium and Hydrochlorothiazide Tablets, USP - Pharmacist: Dispense the Patient Information provided ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL – 100/25 mg – 90 tablets Label-Existing Manufacturing Site Zhejiang Huahai Xunqiao Container Label – 100/25 mg – 90 tablets - Rx only - NDC 43547-424-09 - Losartan Potassium and Hydrochlorothiazide Tablets, USP - Pharmacist: Dispense the Patient Information provided separately ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL – 50/12.5 mg – 90 tablets Label- Alternate Manufacturing Site Zhejiang Huahai Technology Jiangnan Container Label – 50/12.5 mg – 90 tablets - Rx only - NDC 43547-423-09 - Losartan Potassium and Hydrochlorothiazide Tablets, USP - Pharmacist: Dispense the Patient Information provided separately ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL – 100/12.5 mg – 90 tablets Label- Alternate Manufacturing Site Zhejiang Huahai Technology Jiangnan Container Label – 100/12.5 mg – 90 tablets - Rx only - NDC 43547-425-09 - Losartan Potassium and Hydrochlorothiazide Tablets, USP - Pharmacist: Dispense the Patient Information provided ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL – 100/12.5 mg – 90 tablets Label- Alternate Manufacturing Site Zhejiang Huahai Technology Jiangnan Container Label – 100/25 mg – 90 tablets - Rx only - NDC 43547-424-09 - Losartan Potassium and Hydrochlorothiazide Tablets, USP - Pharmacist: Dispense the Patient Information provided separately ...
-
INGREDIENTS AND APPEARANCEProduct Information