Label: DIPHENHYDRAMINE HYDROCHLORIDE injection, solution
- NDC Code(s): 76045-102-10
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
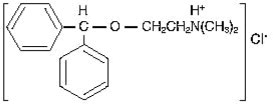
Diphenhydramine hydrochloride is an antihistamine drug having the chemical name 2-(Diphenylmethoxy)-N,N-dimethylethylamine hydrochloride. It occurs as a white, crystalline powder, is freely soluble in water and alcohol and has a molecular weight of 291.82. The molecular formula is C 17H 21NO·HCl and the structural formula is as follows:
Diphenhydramine hydrochloride in the parenteral form is a sterile, pyrogen-free solution. Each mL contains a concentration of 50 mg of diphenhydramine hydrochloride and water for injection, for intramuscular or intravenous use. The solution for parenteral use has been adjusted to a pH between 4 and 6.5 with either sodium hydroxide or hydrochloric acid.
-
CLINICAL PHARMACOLOGY
Diphenhydramine hydrochloride is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines appear to compete with histamine for cell receptor sites on effector cells.
Diphenhydramine hydrochloride in the injectable form has a rapid onset of action. Diphenhydramine hydrochloride is widely distributed throughout the body, including the CNS. A portion of the drug is excreted unchanged in the urine, while the rest is metabolized via the liver. Detailed information on the pharmacokinetics of Diphenhydramine Hydrochloride Injection is not available.
-
INDICATIONS AND USAGE
Diphenhydramine hydrochloride in the injectable form is effective in adults and pediatric patients, other than premature infants and neonates, for the following conditions when diphenhydramine hydrochloride in the oral form is impractical.
Antihistaminic: For amelioration of allergic reactions to blood or plasma, in anaphylaxis as an adjunct to epinephrine and other standard measures after the acute symptoms have been controlled, and for other uncomplicated allergic conditions of the immediate type when oral therapy is impossible or contraindicated.
Motion Sickness: For active treatment of motion sickness.
Antiparkinsonism: For use in parkinsonism, when oral therapy is impossible or contraindicated, as follows: parkinsonism in the elderly who are unable to tolerate more potent agents; mild cases of parkinsonism in other age groups, and in other cases of parkinsonism in combination with centrally acting anticholinergic agents.
-
CONTRAINDICATIONS
Use in Neonates or Premature Infants: This drug should not be used in neonates or premature infants.
Use in Nursing Mothers: Because of the higher risk of antihistamines for infants generally, and for neonates and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
-
WARNINGS
Antihistamines should be used with considerable caution in patients with narrow-angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, symptomatic prostatic hypertrophy, or bladder-neck obstruction.
Local necrosis has been associated with the use of subcutaneous or intradermal use of intravenous diphenhydramine.
-
PRECAUTIONS
General: Diphenhydramine hydrochloride has an atropine-like action and, therefore should be used with caution in patients with a history of bronchial asthma, increased intraocular pressure, hyperthyroidism, cardiovascular disease or hypertension. Use with caution in patients with lower respiratory disease including asthma.
Information for Patients: Patients taking diphenhydramine hydrochloride should be advised that this drug may cause drowsiness and has an additive effect with alcohol.
Patients should be warned about engaging in activities requiring mental alertness such as driving a car or operating appliances, machinery, etc.
Drug Interactions: Diphenhydramine hydrochloride has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc).
MAO inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term studies in animals to determine mutagenic and carcinogenic potential have not been performed.
Pregnancy: Pregnancy Category B: Reproduction studies have been performed in rats and rabbits at doses up to 5 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to diphenhydramine hydrochloride. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pediatric Use: Diphenhydramine should not be used in neonates and premature infants (see CONTRAINDICATIONS).
Diphenhydramine may diminish mental alertness, or in the young pediatric patient, cause excitation. Overdosage may cause hallucinations, convulsions, or death (see WARNINGS and OVERDOSAGE).
See also DOSAGE AND ADMINISTRATION section.
-
ADVERSE REACTIONS
The most frequent adverse reactions are underscored:
- General: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose, and throat.
- Cardiovascular System: Hypotension, headache, palpitations, tachycardia, extrasystoles.
- Hematologic System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
- Nervous System: Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesia, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, neuritis, convulsions.
- GI System: Epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation.
- GU System: Urinary frequency, difficult urination, urinary retention, early menses.
- Respiratory System: Thickening of bronchial secretions, tightness of chest or throat and wheezing, nasal stuffiness.
-
OVERDOSAGE
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in pediatric patients. Atropine-like signs and symptoms; dry mouth; fixed, dilated pupils; flushing; and gastrointestinal symptoms may also occur.
Stimulants should not be used.
Vasopressors may be used to treat hypotension.
-
DOSAGE AND ADMINISTRATION
THIS PRODUCT IS FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION ONLY.
Diphenhydramine hydrochloride in the injectable form is indicated when the oral form is impractical.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
-
HOW SUPPLIED
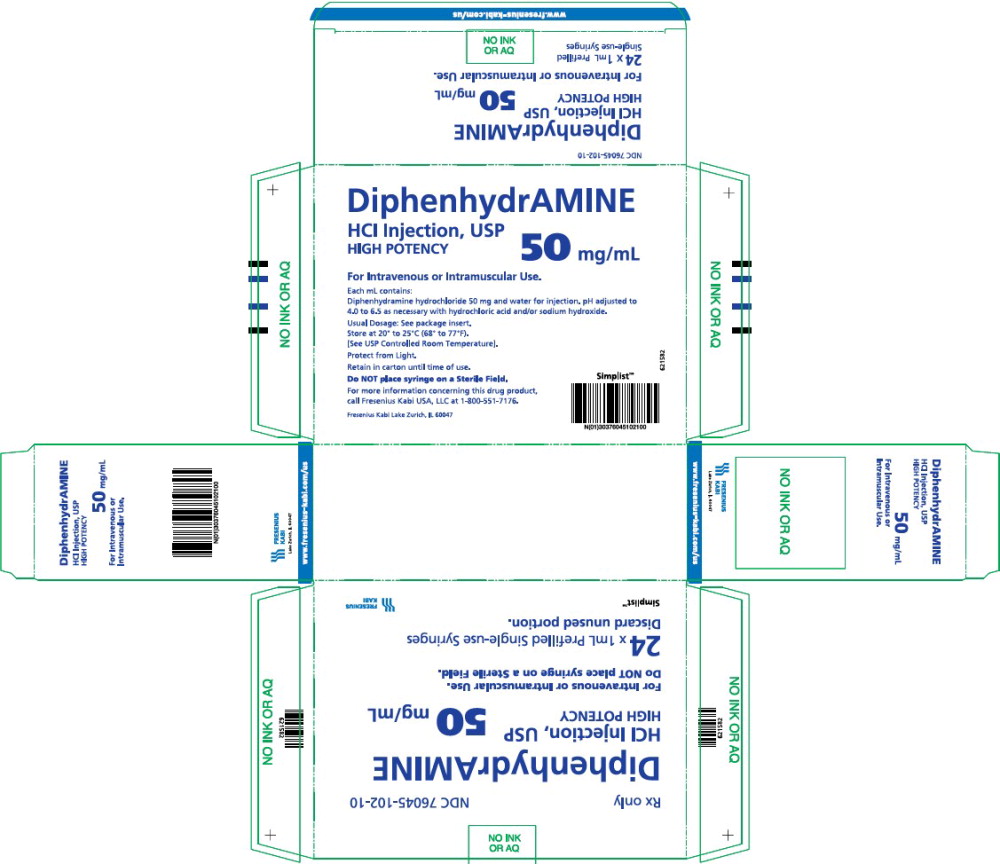
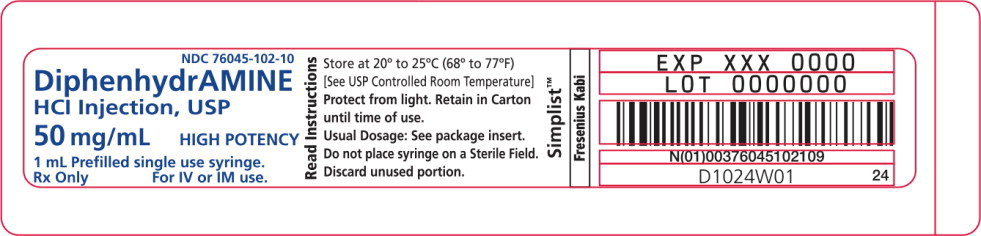
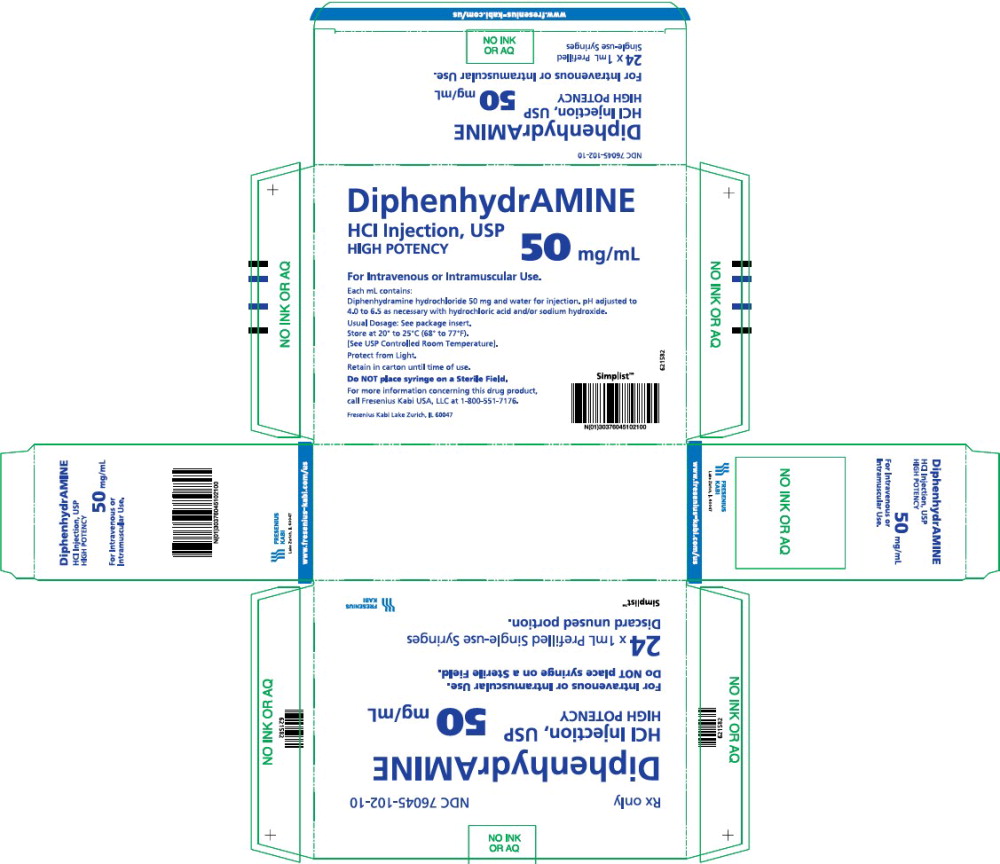
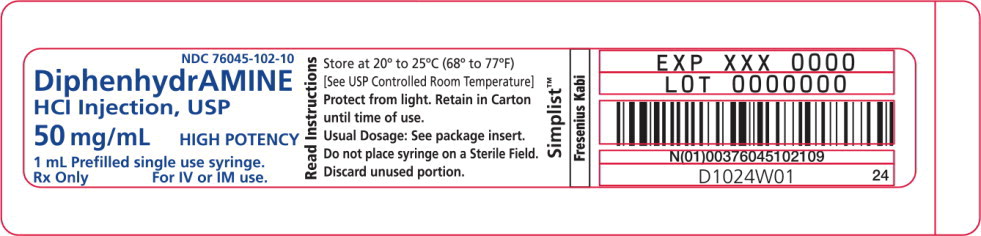
Diphenhydramine Hydrochloride Injection, USP is a clear and colorless solution available as:
50 mg/mL in a 1 mL prefilled single-use syringe.
Available in a carton of twenty-four (24) syringes. NDC 76045-102-10
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature.]
Protect from freezing and light. Retain in carton until time of use.
Do not place syringe on a sterile field.
-
INSTRUCTIONS FOR USE
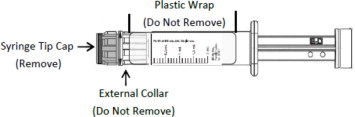
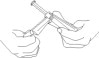
CAUTION: Certain glass syringes may malfunction, break or clog when connected to some Needleless Luer Access Devices (NLADs) and needles. This syringe has a larger internal syringe tip and an external collar (luer collar). The external collar must remain attached to the syringe. Data show that the syringe achieves acceptable connections with the BD Eclipse™ Needle and the Terumo SurGuard2™ Safety Needle and with the following non-center post NLADs: Alaris SMARTSITE™, B-Braun ULTRASITE™, BD-Q SYTE™, Maximum MAX PLUS™, and B-Braun SAFSITE™. The data also show acceptable connections are achieved to the center post ICU Medical CLAVE™. However, spontaneous disconnection of this glass syringe from needles and NLADs with leakage of drug product may occur. Assure that the needle or NLAD is securely attached before beginning the injection. Visually inspect the glass syringe-needle or glass syringe –NLAD connection before and during drug administration. Do not remove the clear plastic wrap around the external collar. (See Figure 1)
- Inspect the outer packaging (blister pack) by verifying:
- blister integrity
- drug name
- drug strength
- dose volume
- route of administration
- expiration date to be sure that the drug has not expired
- sterile field applicability
Do not use if package has been damaged.
- Peel open the paper (top web) of the outer packaging that displays the product information to access the syringe. Do not pop syringe through.
- Bend the plastic part of the outer packaging (thermoform) so as to present the plunger rod for syringe removal. (See Figure 2)
- Perform visual inspection on the syringe by verifying:
- absence of syringe damage
- absence of external particles
- absence of internal particles
- proper drug color
- expiration date to be sure that the drug has not expired
- drug name
- drug strength
- dose volume
- route of administration
- integrity of the plastic wrap around the external collar
- Do not remove plastic wrap around the external collar. Push plunger rod slightly to break the stopper loose while tip cap is still on.
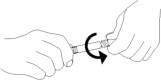
- Do not remove plastic wrap around the external collar. Remove tip cap by twisting it off. (See Figure 3)
- Discard the tip cap.
- Expel air bubble.
- Adjust dose into sterile material (if applicable).
- Connect the syringe to appropriate injection connection depending on route of administration. Before injection, ensure that the syringe is securely attached to the needle or needleless luer access device (NLAD).
- Depress plunger rod to deliver medication. Ensure that pressure is maintained on the plunger rod during the entire administration.
- Remove syringe from NLAD (if applicable) and discard into appropriate receptacle. If delivering the medication with a needle, to prevent needle stick injuries, do not recap needle.
NOTES:
- - All steps must be done sequentially
- - Do not autoclave syringe
- - Do not use this product on a sterile field
- - Do not introduce any other fluid into the syringe at any time
- - This product is for single dose only
For more information concerning this drug, please call Fresenius Kabi USA, LLC at 1-800-551-7176.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
The brand names mentioned in this document are the trademarks of their respective owners.
www.fresenius-kabi.com/us
451609
Rev: November 2018 - Inspect the outer packaging (blister pack) by verifying:
- PACKAGE LABEL - PRINCIPAL DISPLAY – Diphenhydramine 1 mL Carton
- PACKAGE LABEL - PRINCIPAL DISPLAY – Diphenhydramine 1 mL Blister Pack Label
- PACKAGE LABEL - PRINCIPAL DISPLAY – Diphenhydramine 1 mL Syringe Label
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:76045-102 Route of Administration INTRAVENOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76045-102-10 24 in 1 CARTON 03/26/2013 1 1 in 1 BLISTER PACK 1 1 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091526 03/26/2013 Labeler - Fresenius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations Fresenius Kabi, USA LLC 964475045 ANALYSIS(76045-102) , MANUFACTURE(76045-102)