Label: IMVEXXY- estradiol insert
- NDC Code(s): 68308-747-00, 68308-747-08, 68308-747-18, 68308-748-00, view more
- Packager: Mayne Pharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 15, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IMVEXXY safely and effectively. See full prescribing information for IMVEXXY. IMVEXXY® (estradiol vaginal inserts) Initial U.S ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, PROBABLE DEMENTIA, AND BREAST CANCER
Estrogen-Alone Therapy
Endometrial Cancer
There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens. Adding a progestogen to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer. Perform adequate diagnostic measures, including directed or random endometrial sampling when indicated, to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding [see Warnings and Precautions (5.3)].
Cardiovascular Disorders and Probable Dementia
The Women's Health Initiative (WHI) estrogen-alone substudy reported increased risks of stroke and deep vein thrombosis (DVT) in postmenopausal women (50 to 79 years of age) during 7.1 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg]-alone, relative to placebo [see Warnings and Precautions (5.2), and Clinical Studies (14.2)].
The WHI Memory Study (WHIMS) estrogen-alone ancillary study of WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age and older during 5.2 years of treatment with daily CE (0.625 mg)-alone, relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see Warnings and Precautions (5.4), Use in Specific Populations (8.5), and Clinical Studies (14.3)].
Do not use estrogen-alone therapy for the prevention of cardiovascular disease or dementia [see Warnings and Precautions (5.2, 5.4), and Clinical Studies (14.2, 14.3)].
Only daily oral 0.625 mg CE was studied in the estrogen-alone substudy of the WHI. Therefore, the relevance of the WHI findings regarding adverse cardiovascular events and dementia to lower CE doses, other routes of administration, or other estrogen-alone products is not known. Without such data, it is not possible to definitively exclude these risks or determine the extent of these risks for other products. Discuss with your patient the benefits and risks of estrogen-alone therapy, taking into account her individual risk profile.
Prescribe estrogens with or without progestogens at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Estrogen Plus Progestin Therapy
Cardiovascular Disorders and Probable Dementia
The WHI estrogen plus progestin substudy reported increased risks of DVT, pulmonary embolism (PE), stroke and myocardial infarction (MI) in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral CE (0.625 mg) combined with medroxyprogesterone acetate (MPA) [2.5 mg], relative to placebo [see Warnings and Precautions (5.2), and Clinical Studies (14.2)].
The WHIMS estrogen plus progestin ancillary study of the WHI, reported an increased risk of developing probable dementia in postmenopausal women 65 years of age and older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women [see Warnings and Precautions (5.4), Use in Specific Populations (8.5), and Clinical Studies (14.3)].
Do not use estrogen plus progestogen therapy for the prevention of cardiovascular disease or dementia [see Warnings and Precautions (5.2, 5.4), and Clinical Studies (14.2, 14.3)].
Breast Cancer
The WHI estrogen plus progestin substudy demonstrated an increased risk of invasive breast cancer [see Warnings and Precautions (5.3), and Clinical Studies (14.2)].
Only daily oral 0.625 mg CE and 2.5 mg MPA were studied in the estrogen plus progestin substudy of the WHI. Therefore, the relevance of the WHI findings regarding adverse cardiovascular events, dementia and breast cancer to lower CE plus other MPA doses, other routes of administration, or other estrogen plus progestogen products is not known. Without such data, it is not possible to definitively exclude these risks or determine the extent of these risks for other products. Discuss with your patient the benefits and risks of estrogen plus progestogen therapy, taking into account her individual risk profile.
Prescribe estrogens with or without progestogens at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Close -
1 INDICATIONS AND USAGE1.1 Treatment of Moderate to Severe Dyspareunia, a Symptom of Vulvar and Vaginal Atrophy, Due to Menopause
-
2 DOSAGE AND ADMINISTRATIONGenerally, when estrogen is prescribed for a postmenopausal woman with a uterus, consider addition of a progestogen to reduce the risk of endometrial cancer. Generally, a woman without a uterus ...
-
3 DOSAGE FORMS AND STRENGTHSIMVEXXY are small, light pink, tear-shaped, vaginal inserts for manual placement into the vagina. IMVEXXY inserts contain 4 mcg or 10 mcg of estradiol. Each insert is imprinted in white ink on one ...
-
4 CONTRAINDICATIONSIMVEXXY is contraindicated in women with any of the following conditions: Undiagnosed abnormal genital bleeding [see Warning and Precautions (5.3)]. Breast cancer or a history of breast cancer ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risks from Systemic Absorption - IMVEXXY is intended only for vaginal administration. Systemic absorption may occur with the use of IMVEXXY [see Pharmacokinetics (12.3)]. The warnings ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed elsewhere in the labeling: Cardiovascular Disorders [see Boxed Warning, Warnings and Precautions (5.2)]. Malignant Neoplasms [see Boxed ...
-
7 DRUG INTERACTIONSIn vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - IMVEXXY is not indicated for use in pregnancy. There are no data with the use of IMVEXXY in pregnant women; however, epidemiologic studies and meta-analyses have ...
-
10 OVERDOSAGEOverdosage of estrogen may cause nausea, vomiting, breast tenderness, abdominal pain, drowsiness and fatigue, and withdrawal bleeding may occur in women. Treatment of overdose consists of ...
-
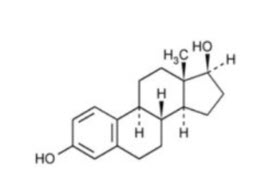
11 DESCRIPTIONIMVEXXY (estradiol vaginal inserts) are small, light pink, tear-shaped, vaginal inserts for manual placement into the vagina. Inserts contain 4 mcg or 10 mcg of estradiol, an estrogen. Each insert ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas ...
-
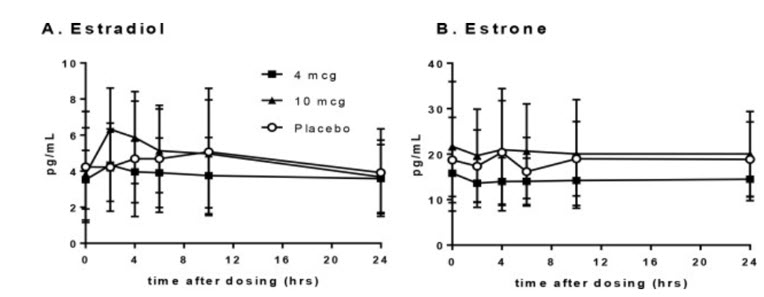
14 CLINICAL STUDIES14.1 Effects on Moderate to Severe Dyspareunia in Postmenopausal Women - The effectiveness and safety of IMVEXXY on moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy due to ...
-
15 REFERENCESRossouw JE, et al. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA. 2007; 297:1465-1477. Hsia J, et al. Conjugated Equine Estrogens and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - IMVEXXY (estradiol vaginal inserts) are small, light pink, tear-shaped inserts for manual placement into the vagina. Inserts contain 4 mcg or 10 mcg of estradiol. Each insert ...
-
17 PATIENT COUNSELING INFORMATIONAdvise women to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Vaginal Bleeding - Inform postmenopausal women of the importance of reporting vaginal ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - IMVEXXY (ĭm vex' ee) (estradiol vaginal inserts) Read this Patient Information before you start using IMVEXXY and each time you get a refill. There may be new ...
-
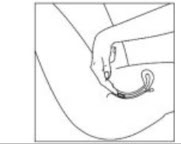
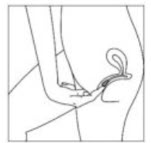
Instructions For UseIMVEXXY® (ĭm vex' ee)(estradiol vaginal inserts)Read this Instructions for Use before you start using IMVEXXY and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare ...
-
PRINCIPAL DISPLAY PANEL - 8 Insert Blister Pack Carton - 4 mcgNDC 68308-747-08 - Rx only - Imvexxy® 4 mcg (estradiol vaginal inserts) FOR VAGINAL USE ONLY - 8 vaginal inserts - mayne pharma
-
PRINCIPAL DISPLAY PANEL - 8 Insert Blister Pack Carton - 10 mcgNDC 68308-748-08 - Rx only - Imvexxy® 10 mcg (estradiol vaginal inserts) FOR VAGINAL USE ONLY - 8 vaginal inserts - mayne pharma
-
INGREDIENTS AND APPEARANCEProduct Information