Label: TOPCARE DAY TIME NITE TIME COLD AND FLU RELIEF- acetaminophen, dextromethorphan hbr, doxylamine succinate, phenylephrine hcl kit
- NDC Code(s): 36800-335-40, 36800-522-40, 36800-567-02
- Packager: Topco Associates LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
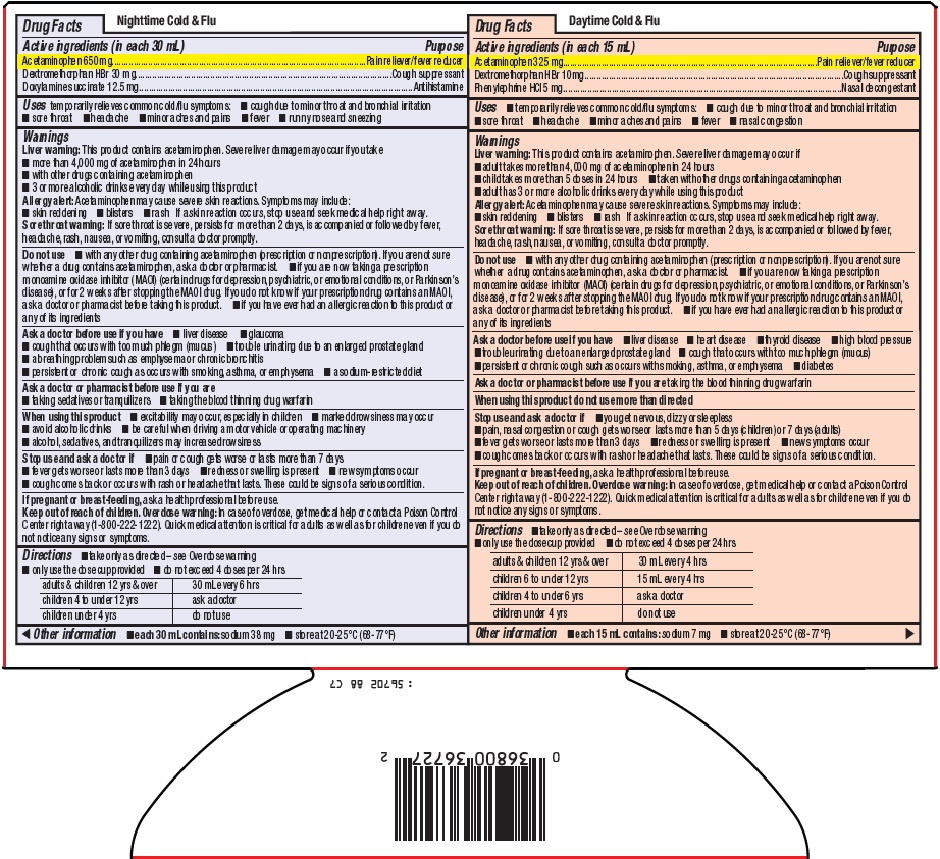
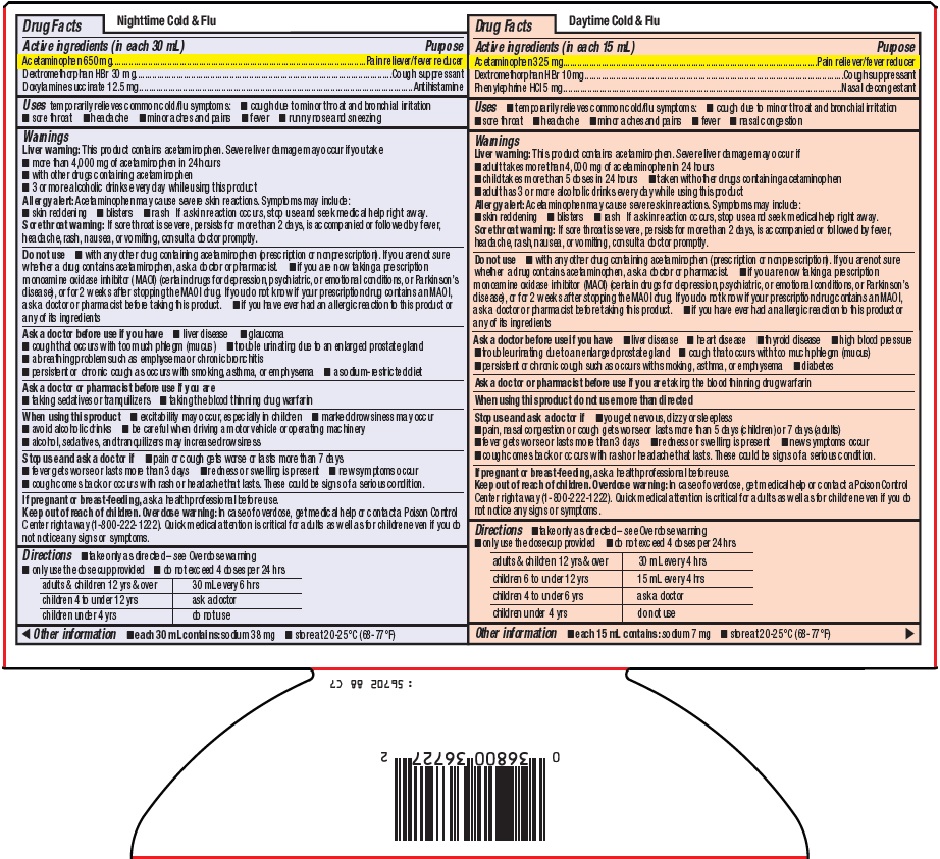
- Nighttime Cold & Flu Active ingredients (in each 30 mL)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- •
- more than 4,000 mg of acetaminophen in 24 hours
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- •
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- •
- liver disease
- •
- glaucoma
- •
- cough that occurs with too much phlegm (mucus)
- •
- trouble urinating due to an enlarged prostate gland
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- persistent or chronic cough as occurs with smoking, asthma, or emphysema
- •
- a sodium-restricted diet
Ask a doctor or pharmacist before use if you are
- •
- taking sedatives or tranquilizers
- •
- taking the blood thinning drug warfarin
When using this product
- •
- excitability may occur, especially in children
- •
- marked drowsiness may occur
- •
- avoid alcoholic drinks
- •
- be careful when driving a motor vehicle or operating machinery
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- Daytime Cold & FluActive ingredients (in each 15 mL)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
- •
- adult takes more than 4,000 mg of acetaminophen in 24 hours
- •
- child takes more than 5 doses in 24 hours
- •
- taken with other drugs containing acetaminophen
- •
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- •
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- •
- liver disease
- •
- heart disease
- •
- thyroid disease
- •
- high blood pressure
- •
- trouble urinating due to an enlarged prostate gland
- •
- cough that occurs with too much phlegm (mucus)
- •
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- •
- diabetes
Stop use and ask a doctor if
- •
- you get nervous, dizzy or sleepless
- •
- pain, nasal congestion or cough gets worse or lasts more than 5 days (children) or 7 days (adults)
- •
- fever gets worse or lasts more than 3 days
- •
- redness or swelling is present
- •
- new symptoms occur
- •
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
SPECIAL VALUE! COMBINATION PACK
TopCare® health
COMPARE TO VICKS® DAYQUIL® ACTIVE INGREDIENTS
MULTI-SYMPTOM RELIEF
Day Time Cold & Flu Relief
PAIN RELIEVER – FEVER REDUCER - ACETAMINOPHEN
COUGH SUPPRESSANT - DEXTROMETHORPHAN HBr
NASAL DECONGESTANT - PHENYLEPHRINE HCl
• Headache, Fever, Sore Throat, Minor Aches & Pains
• Nasal Congestion
• Cough
Alcohol Free
Antihistamine Free
Non-Drowsy
12 FL OZ (355 mL)
ORIGINAL FLAVOR
TopCare® health
COMPARE TO VICKS® NYQUIL® ACTIVE INGREDIENTS
MULTI-SYMPTOM RELIEF
Nite Time Cold & Flu Relief
PAIN RELIEVER – FEVER REDUCER - ACETAMINOPHEN
COUGH SUPPRESSANT - DEXTROMETHORPHAN HBr
ANTIHISTAMINE - DOXYLAMINE SUCCINANATE
• Headache, Fever, Sore Throat, Minor Aches & Pains
• Sneezing, Runny Nose
• Cough
ALCOHOL 10%
12 FL OZ (355 mL)
ORIGINAL FLAVOR
-
INGREDIENTS AND APPEARANCE
TOPCARE DAY TIME NITE TIME COLD AND FLU RELIEF
acetaminophen, dextromethorphan hbr, doxylamine succinate, phenylephrine hcl kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36800-567 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-567-02 1 in 1 CARTON; Type 0: Not a Combination Product 08/07/2012 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 355 mL Part 2 1 BOTTLE 355 mL Part 1 of 2 TOPCARE NITE TIME COLD AND FLU RELIEF
acetaminophen, dextromethorphan hbr, doxylamine succinate solutionProduct Information Item Code (Source) NDC:36800-335 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 30 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 30 mL DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 12.5 mg in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Product Characteristics Color GREEN (clear, bright green) Score Shape Size Flavor FRUIT (anise / cooling menthol aroma) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-335-40 355 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/18/2011 Part 2 of 2 TOPCARE DAY TIME COLD AND FLU RELIEF MULTI SYMPTOM RELIEF
acetaminophen, dextromethorphan hbr, phenylephrine hcl solutionProduct Information Item Code (Source) NDC:36800-522 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg in 15 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 15 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg in 15 mL Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SUCROSE (UNII: C151H8M554) XANTHAN GUM (UNII: TTV12P4NEE) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) Product Characteristics Color ORANGE (clear) Score Shape Size Flavor MENTHOL (with fruit) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-522-40 355 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/17/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/07/2012 Labeler - Topco Associates LLC (006935977)