Label: DECITABINE injection, powder, lyophilized, for solution
- NDC Code(s): 70710-1656-1
- Packager: Zydus Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DECITABINE FOR INJECTION safely and effectively. See full prescribing information for DECITABINE FOR INJECTION. DECITABINE for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDecitabine for injection is indicated for treatment of adult patients with myelodysplastic syndromes (MDS) including previously treated and untreated, de novo and secondary MDS of all ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Pre-Medications and Baseline Testing - Consider pre-medicating for nausea with antiemetics. Conduct baseline laboratory testing: complete blood count (CBC) with ...
-
3 DOSAGE FORMS AND STRENGTHSFor Injection: 50 mg of decitabine as a sterile, white to almost white lyophilized cake or powder in a single-dose vial for reconstitution.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - Fatal and serious myelosuppression occurs in decitabine-treated patients. Myelosuppression (anemia, neutropenia, and thrombocytopenia) is the most frequent cause of ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Myelosuppression [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONSDrug interaction studies with decitabine have not been conducted. In vitro studies in human liver microsomes suggest that decitabine is unlikely to inhibit or induce cytochrome P450 enzymes. In ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from human data, animal studies, and the mechanism of action, decitabine can cause fetal harm when administered to a pregnant woman [see Clinical ...
-
10 OVERDOSAGEThere is no known antidote for overdosage with decitabine. Higher doses are associated with increased myelosuppression including prolonged neutropenia and thrombocytopenia. Standard supportive ...
-
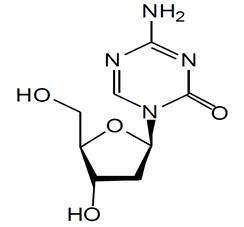
11 DESCRIPTIONDecitabine is a nucleoside metabolic inhibitor. Decitabine is a fine, white to off white powder with the molecular formula of C8H12N4O4 and a molecular weight of 228.21. Its chemical name is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Decitabine is believed to exert its antineoplastic effects after phosphorylation and direct incorporation into DNA and inhibition of DNA methyltransferase, causing ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility - Carcinogenicity studies with decitabine have not been conducted. The mutagenic potential of decitabine was tested in several in ...
-
14 CLINICAL STUDIES14.1 Controlled Trial in Myelodysplastic Syndrome - A randomized open-label, multicenter, controlled trial evaluated 170 adult patients with myelodysplastic syndromes (MDS) meeting ...
-
15 REFERENCESOSHA Hazardous Drugs." OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
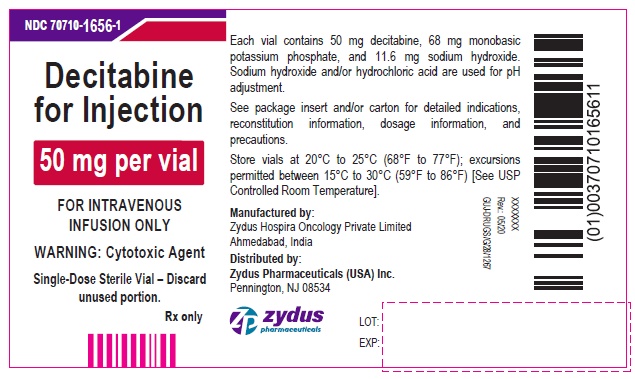
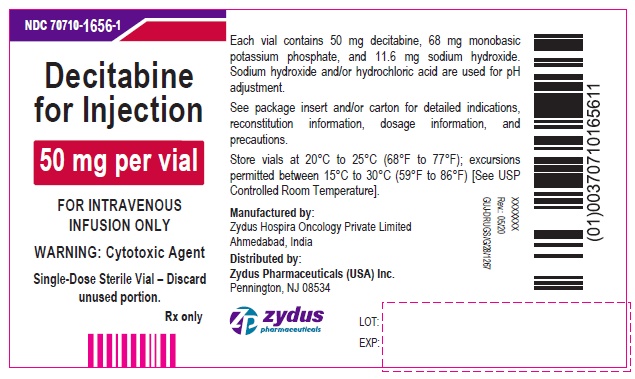
16 HOW SUPPLIED/STORAGE AND HANDLINGDecitabine for injection is a sterile, white to almost white lyophilized cake or powder for intravenous use supplied as: Strength - (mg of decitabine) Pack - NDC - 50 mg/vial ...
-
17 PATIENT COUNSELING INFORMATIONMyelosuppression - Advise patients of the risk of myelosuppression and to report any symptoms of infection, anemia, or bleeding to their healthcare provider as soon as possible. Advise patients ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Zydus Hospira Oncology Private Limited - Ahmedabad, India - Distributed by: Zydus Pharmaceuticals (USA) Inc. Pennington, NJ 08534 - Rev.: 07/20
-
PRINCIPAL DISPLAY PANEL - 50 mg Vial Container LabelNDC 70710-1656-1 - Decitabine for Injection - 50 mg per vial - FOR INTRAVENOUS INFUSION ONLY - WARNING: Cytotoxic Agent - Single-Dose Sterile Vial – Discard unused portion. Rx only
-
PRINCIPAL DISPLAY PANEL - 50 mg Vial CartonNDC 70710-1656-1 - Decitabine for Injection - 50 mg per vial - FOR INTRAVENOUS INFUSION ONLY - WARNING: Cytotoxic Agent - Single-Dose Sterile Vial – Discard unused portion. Rx only - zydus ...
-
INGREDIENTS AND APPEARANCEProduct Information