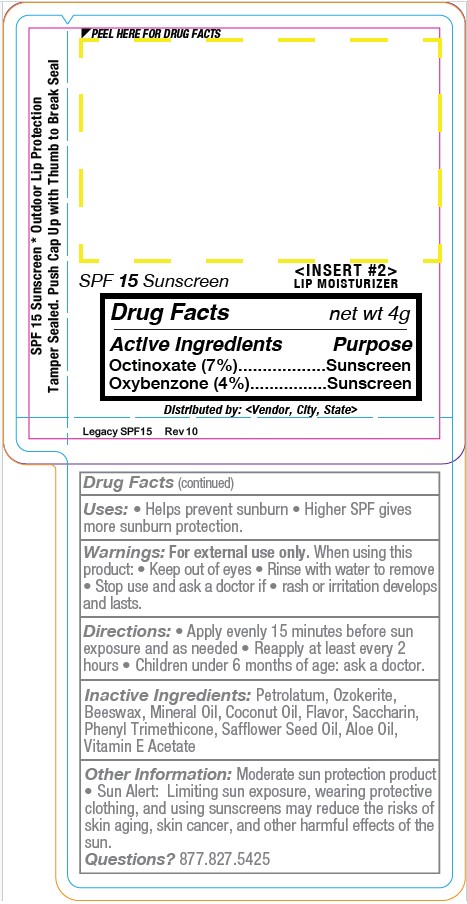
Label: YMLABS- spf15 flavored lip balm stick
- NDC Code(s): 10827-0004-1
- Packager: Yusef Manufacturing Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- WARNINGS
- INSTRUCTIONS FOR USE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
YMLABS
spf15 flavored lip balm stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10827-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.168 g in 4.2 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.294 g in 4.2 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) CERESIN (UNII: Q1LS2UJO3A) WHITE WAX (UNII: 7G1J5DA97F) MINERAL OIL (UNII: T5L8T28FGP) COCONUT OIL (UNII: Q9L0O73W7L) SACCHARIN CALCIUM (UNII: 5101OP7P2I) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) SAFFLOWER OIL (UNII: 65UEH262IS) ALOE (UNII: V5VD430YW9) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10827-0004-1 4.2 g in 1 TUBE; Type 0: Not a Combination Product 12/30/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug part352 12/30/2021 Labeler - Yusef Manufacturing Laboratories (144150674) Registrant - Yusef Manufacturing Laboratories (144150674) Establishment Name Address ID/FEI Business Operations Yusef Manufacturing Laboratories 144150674 manufacture(10827-0004)