Label: RALGRO- zeranol implant
- NDC Code(s): 0061-5026-02, 0061-5026-03
- Packager: Merck Sharp & Dohme Corp.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
INDICATIONS & USAGE
INDICATIONS FOR USE: For increased rate of weight gain and improved feed efficiency in growing beef steers and heifers fed in confinement for slaughter. For increased rate of weight gain in beef calves 2 months of age or older, and in growing beef steers and heifers on pasture (stocker, feeder, and slaughter), and in growing beef steers and heifers in a dry lot. Not approved for repeated implantation (reimplantation) with this or any other cattle ear implant within a single production phase as safety and effectiveness have not been evaluated.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves because effectiveness and safety have not been evaluated.
Do not use in replacement beef heifers after weaning or in bulls, dairy cows or replacement dairy heifers.
-
RESIDUE WARNING

WITHDRAWAL PERIODS AND RESIDUE WARNINGS
No withdrawal period is required when used according to labeling.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has
not been established for this product in pre-ruminating calves.
Do not use in replacement beef heifers after weaning or in dairy cows or replacement dairy heifers. Use in
these cattle may cause drug residues in milk and/or calves born to these cows.
Implant pellets subcutaneously in ear only. Any other location is a violation of Federal law. Do not attempt
salvage of implanted site for human or animal food.
-
SPL UNCLASSIFIED SECTION
Manufactured by a non-sterilizing process.
Restricted Drug (California), Use Only as Directed.
Approved by FDA under NADA # 038-233
Distributed by: Intervet Inc d/b/a Merck Animal Health
Madison, NJ 07940
Copyright © 2022 Merck & Co., Inc., Rahway, NJ, USA and its affiliates.
All rights reserved.
Rev. 03/2023 -
SAFE HANDLING WARNING
USER SAFETY WARNINGS: Not for use in humans. Keep out of reach of children.
ANIMAL SAFETY WARNINGS: Edema of the vulva and udder, teat elongation, rectal and vaginal prolapse, and signs of estrus may occur when heifers are implanted. Delayed testicular development may occur in young males. To avoid difficulty in castration, young males should be castrated at the time of implanting.
-
DOSAGE & ADMINISTRATION
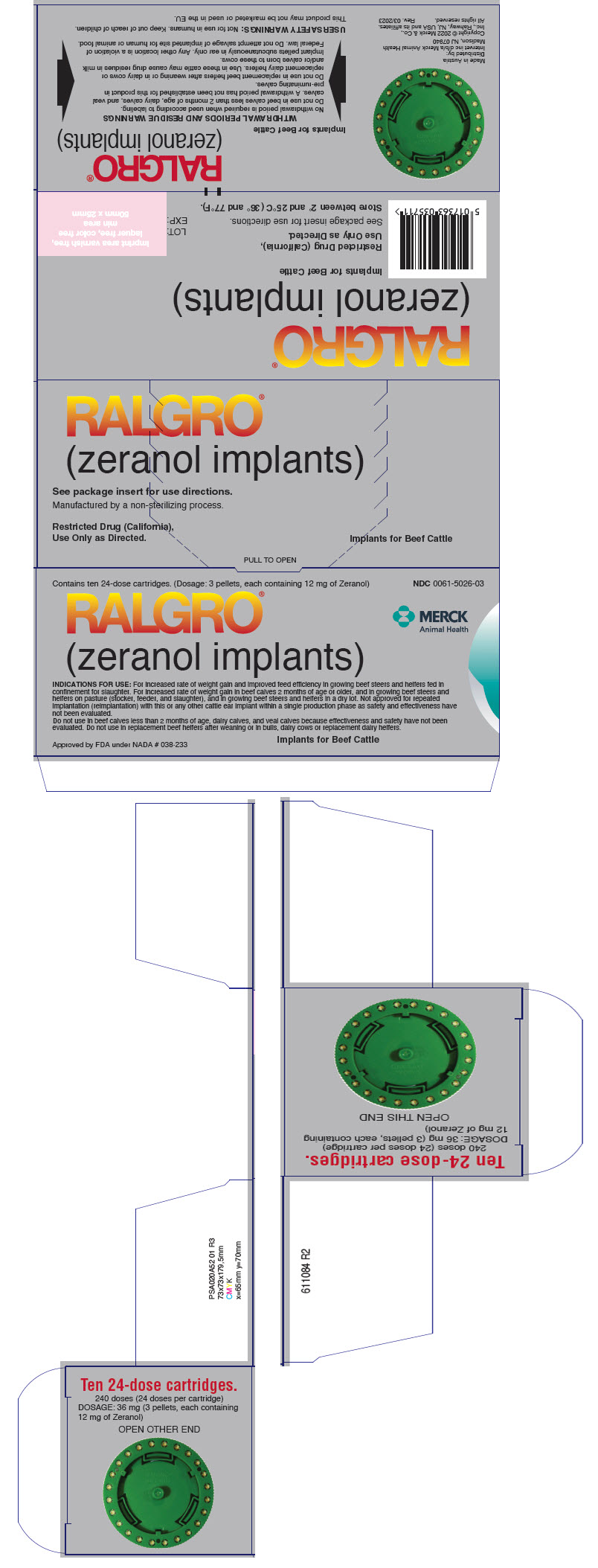
DIRECTIONS: A single pull of the RALOGUN® Pellet Injector trigger delivers one dose (36-mg provided as three 12-mg pellets per chamber).
STEP 1
Each chamber of the plastic RALGRO cartridge contains a full dose of RALGRO implants. Insert the cartridge in the magazine of the RALOGUN pellet injector with the hole in the axle of the cartridge pointed toward the handle of the RALOGUN pellet injector. After snapping into place, rotate cartridge clockwise to ensure proper seating, then check window at top to ensure that pellet chamber is aligned with plunger.
STEP 2
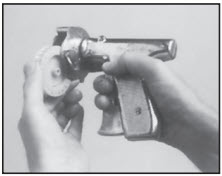
Always use sharp needle. A dull needle tears tissue and makes proper implanting difficult. Be sure that needle is fully seated in RALOGUN pellet injector. This ensures that pellets will be expelled from the needle as RALOGUN plunger is extended. Failure to set the needle could cause the last pellet to be left in the needle and not implanted.
STEP 3
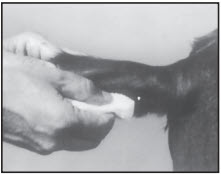
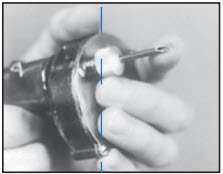
After appropriately restraining the animal to allow access to the ear, cleanse the skin at the implant needle puncture site.
STEP 4
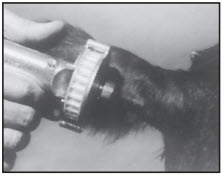
The implant site is subcutaneous, between the skin and cartilage on the back side of the ear and below the midline of the ear. The implant must not be placed closer to the head than the edge of the auricular cartilage ring farthest from the head. The location for insertion of the needle is a point toward the tip of the ear and at least a needle length away from the intended deposition site. Care should be taken to avoid injuring the major blood vessels or cartilage of the ear. Squeeze trigger of the RALOGUN pellet injector to deliver a full dose of RALGRO. Keep trigger depressed while withdrawing the needle to be sure that RALGRO implants stay in place.
STEP 5
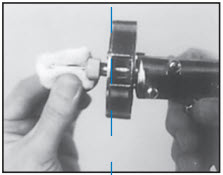
Wipe needle with cotton or gauze moistened with alcohol or other suitable disinfectant. Do not dip needle in solution because solution clinging to inside of needle will cause plugging of needle.
STEP 6
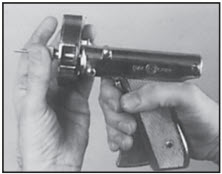
Rotate cartridge in direction of arrow until a new dose is visible and centered in window. The RALOGUN pellet injector is now ready for next implantation.
- STORAGE AND HANDLING
-
SPL UNCLASSIFIED SECTION
QUESTIONS/COMMENTS? For a copy of the Safety Data Sheet or to report side effects, contact Merck Animal Health Technical Services at 1-800-211-3573.
For additional information about reporting side effects for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae
Made in Austria
This product may not be marked or used in the EU.
Approved by FDA under NADA # 038-233
167206 R2
-
PRINCIPAL DISPLAY PANEL - 10 Cartridge Box
Contains ten 24-dose cartridges. (Dosage: 3 pellets, each containing 12 mg of Zeranol)
NDC 0061-5026-03RALGRO®
(zeranol implants)INDICATIONS FOR USE: For increased rate of weight gain and improved feed efficiency in growing beef steers and heifers fed in
confinement for slaughter For increased rate of weight gain in beef calves 2 months of age or older, and in growing beef steers and
heifers on pasture (stoker, feeder, and slaughter), and in growing beef steers and heifers in a dry lot. Not approved for repeated
implantation (reimplantation) with this or any other cattle ear implant within a single production phase as safety and effectiveness have
not been evaluated.
Do not use in beef calves less than 2 months of age, dairy calves, and veal calves because effectiveness and safety have not been
evaluated. Do not use in replacement beef heifers after weaning or in bulls, dairy cows or replacement dairy heifers.Approved by FDA under NADA # 038-233
Implants for Beef Cattle
MERCK
Animal Health
-
INGREDIENTS AND APPEARANCE
RALGRO
zeranol implantProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:0061-5026 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZERANOL (UNII: 76LO2L2V39) (ZERANOL - UNII:76LO2L2V39) ZERANOL 12 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0061-5026-02 1 in 1 CARTRIDGE 1 24 in 1 DOSE PACK 2 NDC:0061-5026-03 10 in 1 CARTRIDGE 2 24 in 1 DOSE PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA038233 01/23/2013 Labeler - Merck Sharp & Dohme Corp. (001317601)