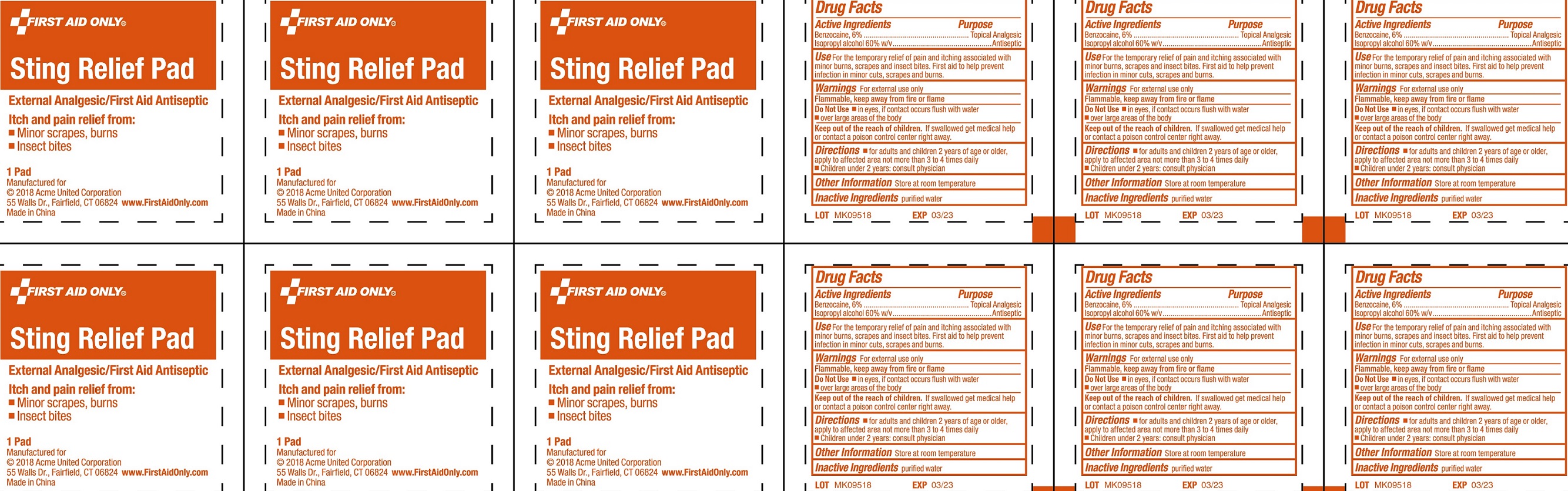
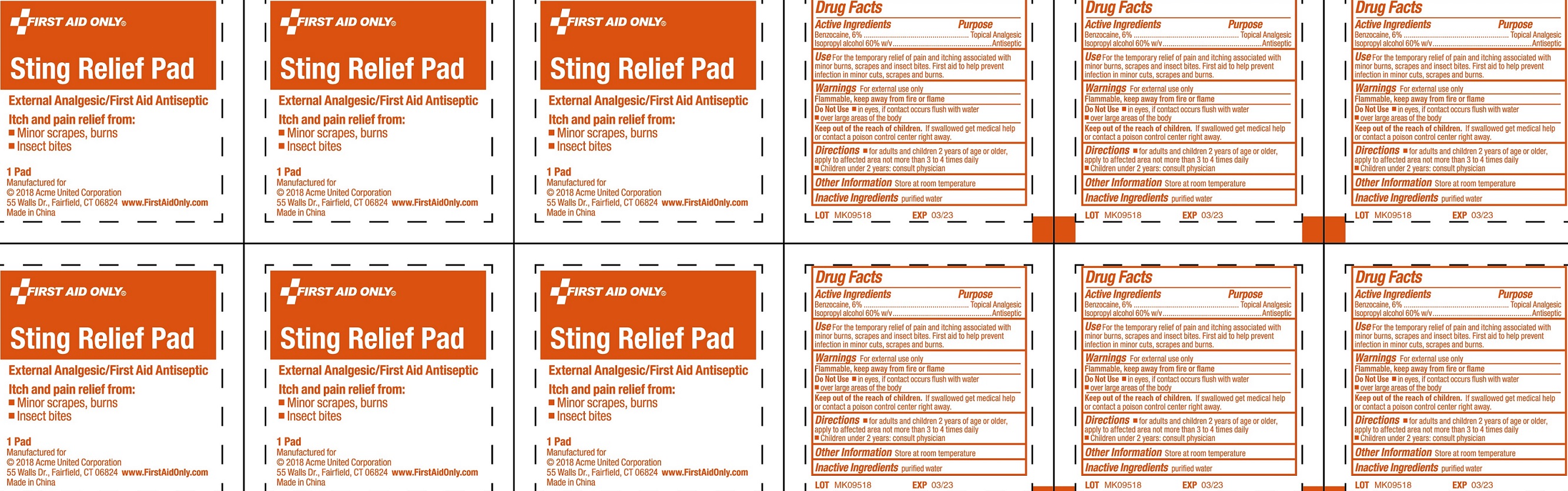
Label: STING RELIEF PAD- benzocaine, isopropyl alcohol swab
- NDC Code(s): 0924-5202-01
- Packager: Acme United Corporation
- This is a repackaged label.
- Source NDC Code(s): 59050-059
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Use
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
STING RELIEF PAD
benzocaine, isopropyl alcohol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0924-5202(NDC:59050-059) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 60 mg in 1 g ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 600 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0924-5202-01 0.34 g in 1 POUCH; Type 0: Not a Combination Product 05/09/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/09/2018 Labeler - Acme United Corporation (001180207) Registrant - Acme United Corporation (001180207) Establishment Name Address ID/FEI Business Operations Acme United Corporation 045924339 relabel(0924-5202) , repack(0924-5202) Establishment Name Address ID/FEI Business Operations Acme United Corporation 080119599 repack(0924-5202) , relabel(0924-5202)