Label: CALAMINE- calamine, pramoxine hcl lotion
- NDC Code(s): 79481-0336-1
- Packager: Meijer Distribution, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Disclaimer
- Adverse Reaction
-
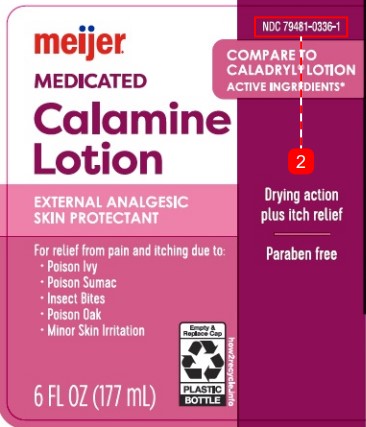
Principal Panel Display
NDC 79481-0336-1
Compare to Caladryl ® Lotion Active Ingredients*
meijer
MEDICATED
Calamine
Lotion
External Analgesic/Skin Protectant
Drying action plus itch relief
Paraben free
For relief from pain and itching due to:
- Poison Ivy
- Poison Sumac
- Insect Bites
- Poison Oak
- Minor Skin Irration
Empty & Replace Cap
Plastic Bottle
how2recycle.info
6 FL OZ (177 mL)
-
INGREDIENTS AND APPEARANCE
CALAMINE
calamine, pramoxine hcl lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79481-0336 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERRIC OXIDE RED (UNII: 1K09F3G675) (FERRIC OXIDE RED - UNII:1K09F3G675) FERRIC OXIDE RED 80 mg in 1 mL PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) BENZYL ALCOHOL (UNII: LKG8494WBH) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LAVENDER OIL (UNII: ZBP1YXW0H8) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ROSEMARY OIL (UNII: 8LGU7VM393) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-0336-1 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/17/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/17/2009 Labeler - Meijer Distribution, Inc (006959555) Registrant - Consumer Product Partners, LLC (119091520) Establishment Name Address ID/FEI Business Operations Consumer Product Partners, LLC 119091514 manufacture(79481-0336)

