Label: ATROPINE SULFATE injection, solution
- NDC Code(s): 0404-9804-20
- Packager: Henry Schein, Inc.
- This is a repackaged label.
- Source NDC Code(s): 63323-580
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONAtropine Sulfate Injection, USP - HIGHLIGHTS OF PRESCRIBING INFORMATION - These highlights do not include all the information needed to use ATROPINE SULFATE INJECTION safely and effectively. See ...
-
Table of ContentsTable of Contents
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ATROPINE SULFATE INJECTION safely and effectively. See full prescribing information for ATROPINE SULFATE INJECTION. ATROPINE ...
-
1 INDICATIONS AND USAGE
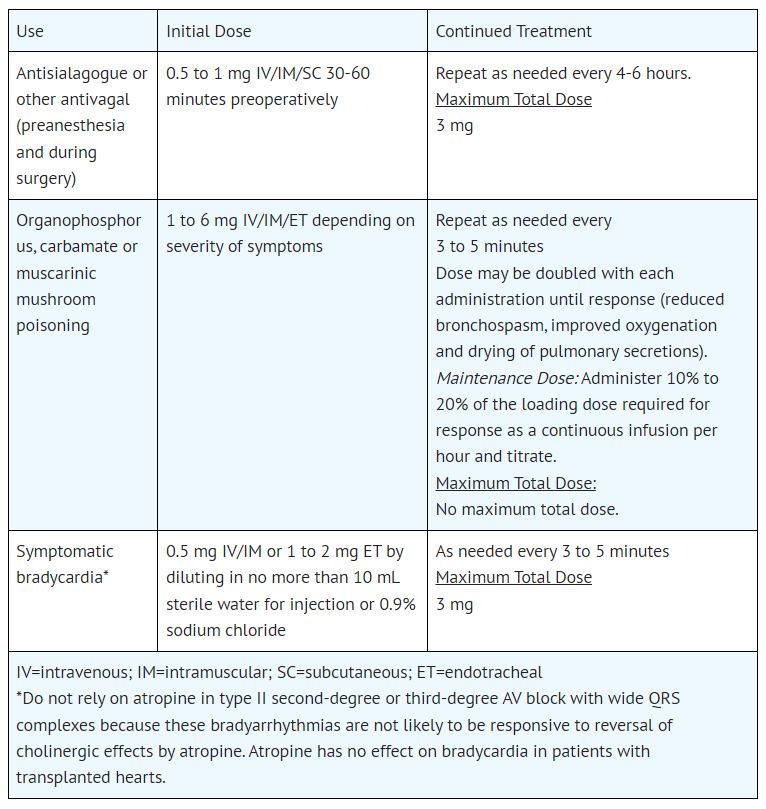
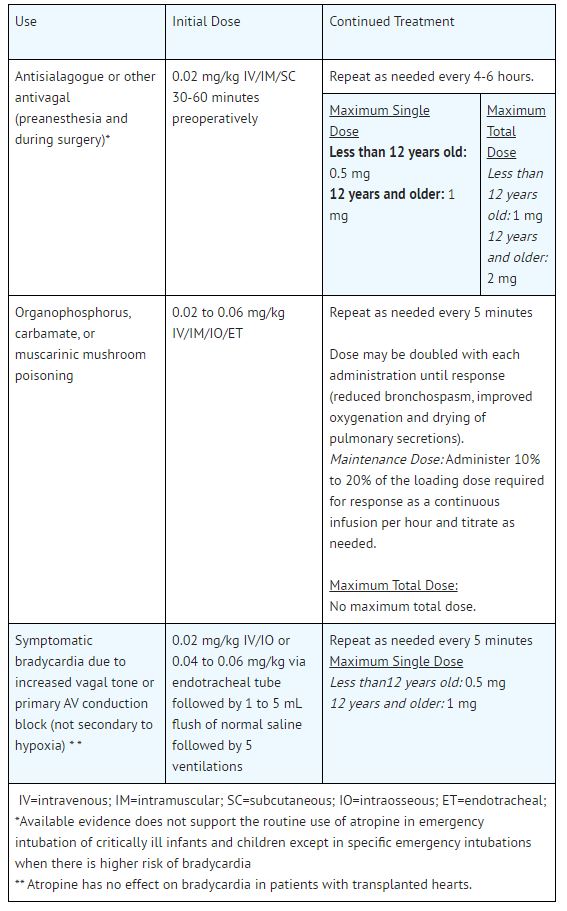
Atropine is indicated for temporary blockade of severe or life threatening muscarinic effects, e.g., as an antisialagogue, an antivagal agent, an antidote for organophosphorus, carbamate, or ...
-
2 DOSAGE AND ADMINISTRATION
2.1 General Administration - Inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not administer unless ...
-
3 DOSAGE FORMS AND STRENGTHS
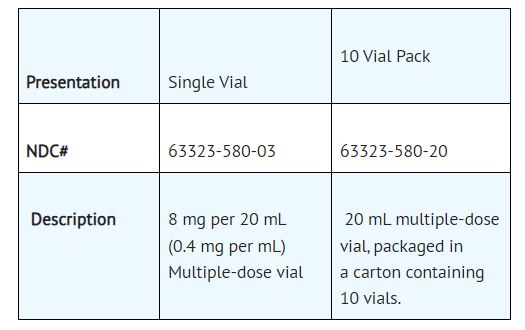
Atropine Sulfate Injection, USP, 8 mg per 20 mL (0.4 mg per mL), is a non-pyrogenic, isotonic, clear solution and is supplied in a multiple dose glass vial.
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity - Atropine may cause anaphylaxis. 5.2 Worsening of Ischemic Heart Disease - In patients with ischemic heart disease, the total dose should be restricted to 2 to 3 mg (maximum ...
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in labeling: Hypersensitivity (5.1) Worsening of Ischemic Heart Disease (5.2) Acute Glaucoma (5.3) Pyloric Obstruction (5.4) Complete ...
-
7 DRUG INTERACTIONS
7.1 Mexiletine - Atropine Sulfate Injection decreased the rate of mexiletine absorption without altering the relative oral bioavailability; this delay in mexiletine absorption was reversed by ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Limited available data with Atropine Sulfate Injection use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes (see ...
-
10 OVERDOSAGE
Excessive dosing may cause palpitation, dilated pupils, difficulty in swallowing, hot dry skin, thirst, dizziness, restlessness, tremor, fatigue and ataxia. Toxic doses lead to restlessness and ...
-
11 DESCRIPTION
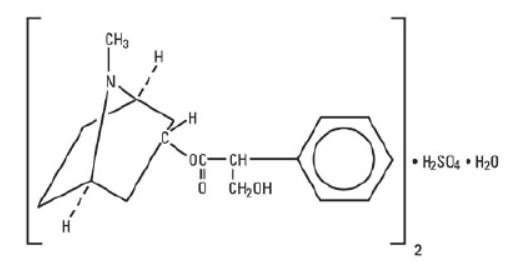
Atropine Sulfate Injection, USP is a sterile, nonpyrogenic, isotonic, clear solution of atropine sulfate in water for injection with sodium chloride sufficient to render the solution isotonic. It ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Atropine is an antimuscarinic agent since it antagonizes the muscarine-like actions of acetylcholine and other choline esters. Atropine inhibits the muscarinic actions ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies have not been performed to evaluate the carcinogenic or mutagenic potential of atropine or its potential to affect fertility ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Atropine Sulfate Injection, USP is a non-pyrogenic, isotonic, clear solution and is supplied as follows: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. After initial ...
-
Sample Package Label

-
INGREDIENTS AND APPEARANCEProduct Information