Label: NOXZEMA ANTIBLEMISH- salicylic acid cloth
- NDC Code(s): 64942-1259-1
- Packager: Conopco Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
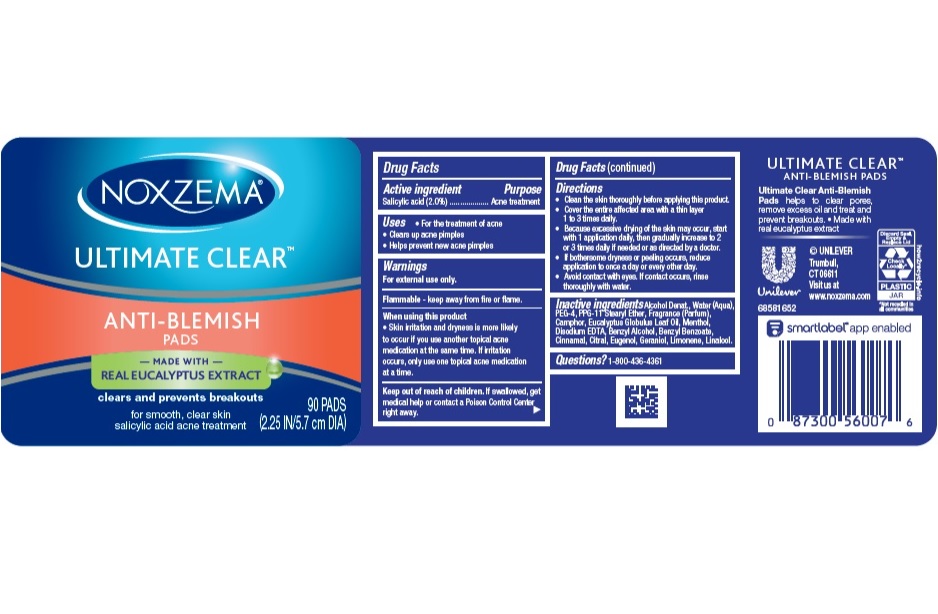
- NOXZEMA ULTIMATE CLEAR ANIT-BLEMISH PADS - (Salicylic Acid) lotion
- Drug Facts
- Purpose
- Uses
- Warnings
-
Directions
- Clean the skin thoroughly before applying product
- Cover the entire affected area with a thin layer and rinse thoroughly one to three times daily
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily, if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Avoid contact with eyes. If contact occurs, rinse thoroughly with water.
- Inactive ingredients
- Questions?
- Packaging
-
INGREDIENTS AND APPEARANCE
NOXZEMA ANTIBLEMISH
salicylic acid clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64942-1259 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.02 g in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) BENZYL ALCOHOL (UNII: LKG8494WBH) CINNAMALDEHYDE (UNII: SR60A3XG0F) GERANIOL (UNII: L837108USY) LIMONENE, (+)- (UNII: GFD7C86Q1W) BENZYL BENZOATE (UNII: N863NB338G) EUGENOL (UNII: 3T8H1794QW) LINALOOL, (+/-)- (UNII: D81QY6I88E) WATER (UNII: 059QF0KO0R) POLYETHYLENE GLYCOL 200 (UNII: R95B8J264J) PPG-11 STEARYL ETHER (UNII: S4G2J0Y0LG) EDETATE DISODIUM (UNII: 7FLD91C86K) MENTHOL (UNII: L7T10EIP3A) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) CITRAL (UNII: T7EU0O9VPP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64942-1259-1 0.0284 g in 1 PACKET; Type 0: Not a Combination Product 09/01/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 09/01/2012 Labeler - Conopco Inc. (001375088)