Label: DIVALPROEX SODIUM capsule, coated pellets
- NDC Code(s): 17856-0109-1
- Packager: ATLANTIC BIOLOGICALS CORP.
- This is a repackaged label.
- Source NDC Code(s): 68382-106
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DIVALPROEX SODIUM DELAYED-RELEASE CAPSULES (SPRINKLE) safely and effectively. See full prescribing information for DIVALPROEX SODIUM ...These highlights do not include all the information needed to use DIVALPROEX SODIUM DELAYED-RELEASE CAPSULES (SPRINKLE) safely and effectively. See full prescribing information for DIVALPROEX SODIUM DELAYED-RELEASE CAPSULES (SPRINKLE).
DIVALPROEX SODIUM delayed-release capsules (sprinkle), for oral use
Initial U.S. Approval: 1989WARNING: LIFE THREATENING ADVERSE REACTIONS
See full prescribing information for complete boxed warning.
- Hepatotoxicity, including fatalities, usually during the first 6 months of treatment. Children under the age of two years and patients with mitochondrial disorders are at higher risk. Monitor patients closely, and perform serum liver testing prior to therapy and at frequent intervals thereafter ( 5.1)
- Fetal Risk, particularly neural tube defects other major malformations, and decreased IQ ( 5.2, 5.3, 5.4)
- Pancreatitis, including fatal hemorrhagic cases ( 5.5)
INDICATIONS AND USAGE
Divalproex sodium delayed-release capsules (sprinkle) are anti-epileptic drug indicated for:
- Monotherapy and adjunctive therapy of complex partial seizures and simple and complex absence seizures; adjunctive therapy in patients with multiple seizure types that include absence seizures ( 1)
DOSAGE AND ADMINISTRATION
- Divalproex sodium delayed-release capsules (sprinkle) may be swallowed whole or the contents may be sprinkled on soft food. The drug/food mixture should be swallowed immediately (avoid chewing) ( 2.2)
- Safety of doses above 60 mg/kg/day is not established ( 2.1), ( 2.2)
- Complex Partial Seizures: Start at 10 to 15 mg/kg/day, increasing at 1 week intervals by 5 to 10 mg/kg/day to achieve optimal clinical response; if response is not satisfactory, check valproate plasma level; see full prescribing information for conversion to monotherapy ( 2.1)
- Absence Seizures: Start at 15 mg/kg/day, increasing at 1 week intervals by 5 to 10 mg/kg/day until seizure control or limiting side effects ( 2.1)
DOSAGE FORMS AND STRENGTHS
Capsules: 125 mg ( 3)
CONTRAINDICATIONS
- Hepatic disease or significant hepatic dysfunction ( 4, 5.1)
- Known mitochondrial disorders caused by mutations in mitochondrial DNA polymerase γ (POLG) ( 4, 5.1)
- Suspected POLG-related disorder in children under two years of age ( 4, 5.1)
- Known hypersensitivity to the drug ( 4, 5.12)
- Urea cycle disorders ( 4, 5.6)
WARNINGS AND PRECAUTIONS
- Hepatotoxicity; evaluate high risk populations and monitor serum liver tests ( 5.1)
- Birth defects and decreased IQ following in uteroexposure; only use to treat pregnant women with epilepsy if other medications are unacceptable; should not be administered to a woman of childbearing potential unless essential ( 5.2, 5.3, 5.4)
- Pancreatitis; divalproex sodium delayed-release capsules (sprinkle) should ordinarily be discontinued ( 5.5)
- Suicidal behavior or ideation; Antiepileptic drugs, including divalproex sodium increase the risk of suicidal thoughts or behavior ( 5.7)
- Bleeding and other hematopoietic disorders; monitor platelet counts and coagulation tests ( 5.8)
- Hyperammonemia and hyperammonemic encephalopathy; measure ammonia level if unexplained lethargy and vomiting or changes in mental status, and also with concomitant topiramate use; consider discontinuation of valproate therapy ( 5.6, 5.9, 5.10)
- Hypothermia; Hypothermia has been reported during valproate therapy with or without associated hyperammonemia. This adverse reaction can also occur in patients using concomitant topiramate ( 5.11)
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan hypersensitivity reaction; discontinue divalproex sodium sprinkle capsules ( 5.12)
- Somnolence in the elderly can occur. Divalproex sodium delayed-release capsules (sprinkle) dosage should be increased slowly and with regular monitoring for fluid and nutritional intake ( 5.14)
ADVERSE REACTIONS
Most common adverse reactions (reported >5%) are abdominal pain, alopecia, amblyopia/blurred vision, amnesia, anorexia, asthenia, ataxia, bronchitis, constipation, depression, diarrhea, diplopia, dizziness, dyspnea, dyspepsia, ecchymosis, emotional lability, fever, flu syndrome, headache, increased appetite, infection, insomnia, nausea, nervousness, nystagmus, thrombocytopenia, somnolence, vomiting, tremor, weight gain, weight loss, peripheral edema, pharyngitis, rhinitis, thinking abnormal, tinnitus ( 6.1)
- The safety and tolerability of valproate in pediatric patients were shown to be comparable to those in adults ( 8.4).
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Hepatic enzyme-inducing drugs (e.g., phenytoin, carbamazepine, phenobarbital, primidone, rifampin) can increase valproate clearance, while enzyme inhibitors (e.g., felbamate) can decrease valproate clearance. Therefore increased monitoring of valproate and concomitant drug concentrations and dosage adjustment are indicated whenever enzyme-inducing or inhibiting drugs are introduced or withdrawn ( 7.1)
- Aspirin, carbapenem antibiotics, estrogen-containing hormonal contraceptives: Monitoring of valproate concentrations is recommended ( 7.1)
- Coadministration of valproate can affect the pharmacokinetics of other drugs (e.g., diazepam, ethosuximide, lamotrigine, phenytoin) by inhibiting their metabolism or protein binding displacement ( 7.2)
- Patients stabilized on rufinamide should begin valproate therapy at a low dose, and titrate to clinically effective dose ( 7.2)
- Dosage adjustment of amitriptyline/nortriptyline, propofol, warfarin, and zidovudine may be necessary if used concomitantly with divalproex sodium delayed-release capsules (sprinkle) ( 7.2)
- Topiramate: Hyperammonemia and encephalopathy ( 5.10, 7.3)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Divalproex sodium delayed-release capsules (sprinkle) can cause congenital malformations including neural tube defects and decreased IQ ( 5.2, 5.3, 8.1)
- Pediatric: Children under the age of two years are at considerably higher risk of fatal hepatotoxicity ( 5.1, 8.4)
- Geriatric: Reduce starting dose; increase dosage more slowly; monitor fluid and nutritional intake, and somnolence ( 5.14, 8.5)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2021
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: LIFE THREATENING ADVERSE REACTIONS
1 INDICATIONS AND USAGE
1.1 Epilepsy
1.2 Important Limitations
2 DOSAGE AND ADMINISTRATION
2.1 Epilepsy
2.2 General Dosing Advice
2.3 Dosing in Patients Taking Rufinamide
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity
5.2 Birth Defects
5.3 Decreased IQ Following in utero Exposure
5.4 Use in Women of Childbearing Potential
5.5 Pancreatitis
5.6 Urea Cycle Disorders
5.7 Suicidal Behavior and Ideation
5.8 Bleeding and Other Hematopoietic Disorders
5.9 Hyperammonemia
5.10 Hyperammonemia and Encephalopathy associated with Concomitant Topiramate Use
5.11 Hypothermia
5.12 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity Reactions
5.13 Interaction with Carbapenem Antibiotics
5.14 Somnolence in the Elderly
5.15 Monitoring: Drug Plasma Concentration
5.16 Effect on Ketone and Thyroid Function Tests
5.17 Effect on HIV and CMV Viruses Replication
5.18 Medication Residue in the Stool
6 ADVERSE REACTIONS
6.1 Epilepsy
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effects of Coadministered Drugs onValproate Clearance
7.2 Effects of Valproate on Other Drugs
7.3 Topiramate
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Effect of Disease
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
14 CLINICAL STUDIES
14.1 Epilepsy
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)Hepatotoxicity - General Population:Hepatic failure resulting in fatalities has occurred in patients receiving valproate and its derivatives. These incidents usually have occurred during the first ...
WARNING: LIFE THREATENING ADVERSE REACTIONS
CloseGeneral Population:Hepatic failure resulting in fatalities has occurred in patients receiving valproate and its derivatives. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months [see WARNINGS AND PRECAUTIONS ( 5.1)].
Children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those on multiple anticonvulsants, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease. When divalproex sodium delayed-release capsules (sprinkle) are used in this patient group, they should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. The incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
Patients with Mitochondrial Disease:There is an increased risk of valproate-induced acute liver failure and resultant deaths in patients with hereditary neurometabolic syndromes caused by DNA mutations of the mitochondrial DNA Polymerase γ (POLG) gene (e.g. Alpers Huttenlocher Syndrome). Divalproex sodium delayed-release capsules (Sprinkle) are contraindicated in patients known to have mitochondrial disorders caused by POLG mutations and children under two years of age who are clinically suspected of having a mitochondrial disorder [see CONTRAINDICATIONS ( 4)]. In patients over two years of age who are clinically suspected of having a hereditary mitochondrial disease, divalproex sodium delayed-release capsules (sprinkle) should only be used after other anticonvulsants have failed. This older group of patients should be closely monitored during treatment with divalproex sodium delayed-release capsules (sprinkle) for the development of acute liver injury with regular clinical assessments and serum liver testing. POLG mutation screening should be performed in accordance with current clinical practice [see WARNINGS AND PRECAUTIONS ( 5.1)].
Fetal Risk
Valproate can cause major congenital malformations, particularly neural tube defects (e.g., spina bifida). In addition, valproate can cause decreased IQ scores following in uteroexposure.
Valproate should only be used to treat pregnant women with epilepsy if other medications have failed to control their symptoms or are otherwise unacceptable.
Valproate should not be administered to a woman of childbearing potential unless the drug is essential to the management of her medical condition. This is especially important when valproate use is considered for a condition not usually associated with permanent injury or death (e.g., migraine). Women should use effective contraception while using valproate [see WARNINGS AND PRECAUTIONS ( 5.2, 5.3, 5.4)].
A Medication Guide describing the risks of valproate is available for patients [see PATIENT COUNSELING INFORMATION ( 17)].
Pancreatitis
Cases of life-threatening pancreatitis have been reported in both children and adults receiving valproate. Some of the cases have been described as hemorrhagic with a rapid progression from initial symptoms to death. Cases have been reported shortly after initial use as well as after several years of use. Patients and guardians should be warned that abdominal pain, nausea, vomiting and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, valproate should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated [see WARNINGS AND PRECAUTIONS ( 5.5)].
-
1 INDICATIONS AND USAGE1.1 Epilepsy - Divalproex sodium delayed-release capsules (sprinkle) are indicated as monotherapy and adjunctive therapy in the treatment of adult patients and pediatric patients down to the ...
1.1 Epilepsy
Divalproex sodium delayed-release capsules (sprinkle) are indicated as monotherapy and adjunctive therapy in the treatment of adult patients and pediatric patients down to the age of 10 years with complex partial seizures that occur either in isolation or in association with other types of seizures. Divalproex sodium delayed-release capsules (sprinkle) are also indicated for use as sole and adjunctive therapy in the treatment of simple and complex absence seizures, and adjunctively in patients with multiple seizure types that include absence seizures.
Simple absence is defined as very brief clouding of the sensorium or loss of consciousness accompanied by certain generalized epileptic discharges without other detectable clinical signs. Complex absence is the term used when other signs are also present.
Close1.2 Important Limitations
Because of the risk to the fetus of decreased IQ, neural tube defects, and other major congenital malformations, which may occur very early in pregnancy, valproate should not be administered to a woman of childbearing potential unless the drug is essential to the management of her medical condition [ see WARNINGS AND PRECAUTIONS( 5.2, 5.3, 5.4), USE IN SPECIFIC POPULATIONS ( 8.1), AND PATIENT COUNSELING INFORMATION ( 17)].
-
2 DOSAGE AND ADMINISTRATION2.1 Epilepsy - Divalproex sodium delayed-release capsules (sprinkle) are administered orally. As divalproex sodium dosage is titrated upward, concentrations of clonazepam, diazepam ...
2.1 Epilepsy
Divalproex sodium delayed-release capsules (sprinkle) are administered orally. As divalproex sodium dosage is titrated upward, concentrations of clonazepam, diazepam, ethosuximide, lamotrigine, tolbutamide, phenobarbital, carbamazepine, and/or phenytoin may be affected [see DRUG INTERACTIONS ( 7.2) ] .
Complex Partial Seizures
For adults and children 10 years of age or older.
Monotherapy (Initial Therapy)
Divalproex sodium has not been systematically studied as initial therapy. Patients should initiate therapy at 10 to 15 mg/kg/day. The dosage should be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made.
The probability of thrombocytopenia increases significantly at total trough valproate plasma concentrations above 110 mcg/mL in females and 135 mcg/mL in males. The benefit of improved seizure control with higher doses should be weighed against the possibility of a greater incidence of adverse reactions.
Conversion to Monotherapy
Patients should initiate therapy at 10 to 15 mg/kg/day. The dosage should be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made.
Concomitant antiepilepsy drug (AED) dosage can ordinarily be reduced by approximately 25% every 2 weeks. This reduction may be started at initiation of divalproex sodium therapy, or delayed by 1 to 2 weeks if there is a concern that seizures are likely to occur with a reduction. The speed and duration of withdrawal of the concomitant AED can be highly variable, and patients should be monitored closely during this period for increased seizure frequency.
Adjunctive Therapy
Divalproex sodium may be added to the patient's regimen at a dosage of 10 to 15 mg/kg/day. The dosage may be increased by 5 to 10 mg/kg/week to achieve optimal clinical response. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg/day. If satisfactory clinical response has not been achieved, plasma levels should be measured to determine whether or not they are in the usually accepted therapeutic range (50 to 100 mcg/mL). No recommendation regarding the safety of valproate for use at doses above 60 mg/kg/day can be made. If the total daily dose exceeds 250 mg, it should be given in divided doses.
In a study of adjunctive therapy for complex partial seizures in which patients were receiving either carbamazepine or phenytoin in addition to valproate, no adjustment of carbamazepine or phenytoin dosage was needed [see CLINICAL STUDIES(14) ]. However, since valproate may interact with these or other concurrently administered AEDs as well as other drugs, periodic plasma concentration determinations of concomitant AEDs are recommended during the early course of therapy [see DRUG INTERACTIONS ( 7) ].
Simple and Complex Absence Seizures
The recommended initial dose is 15 mg/kg/day, increasing at one week intervals by 5 to 10 mg/kg/day until seizures are controlled or side effects preclude further increases. The maximum recommended dosage is 60 mg/kg/day. If the total daily dose exceeds 250 mg, it should be given in divided doses.
A good correlation has not been established between daily dose, serum concentrations, and therapeutic effect. However, therapeutic valproate serum concentrations for most patients with absence seizures are considered to range from 50 to 100 mcg/mL. Some patients may be controlled with lower or higher serum concentrations [see CLINICAL PHARMACOLOGY ( 12.3) ].
As the divalproex sodium delayed-release capsules (sprinkle) dosage is titrated upward, blood concentrations of phenobarbital and/or phenytoin may be affected [see DRUG INTERACTIONS ( 7.2) ].
Antiepilepsy drugs should not be abruptly discontinued in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life.
In epileptic patients previously receiving valproic acid therapy, divalproex sodium delayed-release capsules (sprinkle) should be initiated at the same daily dose and dosing schedule. After the patient is stabilized on divalproex sodium delayed-release capsules (sprinkle), a dosing schedule of two or three times a day may be elected in selected patients.
2.2 General Dosing Advice
Due to a decrease in unbound clearance of valproate and possibly a greater sensitivity to somnolence in the elderly, the starting dose should be reduced in these patients. Dosage should be increased more slowly and with regular monitoring for fluid and nutritional intake, dehydration, somnolence, and other adverse reactions. Dose reductions or discontinuation of valproate should be considered in patients with decreased food or fluid intake and in patients with excessive somnolence. The ultimate therapeutic dose should be achieved on the basis of both tolerability and clinical response [see WARNINGS AND PRECAUTIONS ( 5.14) , USE IN SPECIFIC POPULATIONS ( 8.5) and CLINICAL PHARMACOLOGY ( 12.3) ].
Dose-Related Adverse Reactions
The frequency of adverse effects (particularly elevated liver enzymes and thrombocytopenia) may be dose-related. The probability of thrombocytopenia appears to increase significantly at total valproate concentrations of ≥ 110 mcg/mL (females) or ≥ 135 mcg/mL (males) [see WARNINGS AND PRECAUTIONS ( 5.8) ]. The benefit of improved therapeutic effect with higher doses should be weighed against the possibility of a greater incidence of adverse reactions.
G.I. Irritation
Patients who experience G.I. irritation may benefit from administration of the drug with food or by slowly building up the dose from an initial low level.
Administration of Sprinkle Capsules
Divalproex sodium delayed-release capsules (sprinkle) may be swallowed whole or may be administered by carefully opening the capsule and sprinkling the entire contents on a small amount (teaspoonful) of soft food such as applesauce or pudding. The drug/food mixture should be swallowed immediately (avoid chewing) and not stored for future use. Each capsule is oversized to allow ease of opening.
Close2.3 Dosing in Patients Taking Rufinamide
Patients stabilized on rufinamide before being prescribed valproate should begin valproate therapy at a low dose, and titrate to a clinically effective dose [see Drug Interactions ( 7.2)] .
-
3 DOSAGE FORMS AND STRENGTHSDivalproex sodium delayed-release capsules, USP (sprinkle) are for oral administration. Divalproex sodium delayed-release capsules, USP (sprinkle) contain specially coated particles of ...Close
Divalproex sodium delayed-release capsules, USP (sprinkle) are for oral administration.
Divalproex sodium delayed-release capsules, USP (sprinkle) contain specially coated particles of divalproex sodium equivalent to 125 mg of valproic acid in a hard gelatin capsule.
-
4 CONTRAINDICATIONSDivalproex sodium delayed-release capsules (sprinkle) should not be administered to patients with hepatic disease or significant hepatic dysfunction [see - WARNINGS AND PRECAUTIONS ...Close
- Divalproex sodium delayed-release capsules (sprinkle) should not be administered to patients with hepatic disease or significant hepatic dysfunction [see WARNINGS AND PRECAUTIONS ( 5.1) ].
- Divalproex sodium delayed-release capsules (sprinkle) are contraindicated in patients known to have mitochondrial disorders caused by mutations in mitochondrial DNA polymerase γ (POLG; e.g., Alpers-Huttenlocher Syndrome) and children under two years of age who are suspected of having a POLG-related disorder [see WARNINGS AND PRECAUTIONS ( 5.1)].
- Divalproex sodium delayed-release capsules (sprinkle) are contraindicated in patients with known hypersensitivity to the drug [see WARNINGS AND PRECAUTIONS ( 5.12) ].
- Divalproex sodium delayed-release capsules (sprinkle) are contraindicated in patients with known urea cycle disorders [see WARNINGS AND PRECAUTIONS ( 5.6) ].
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity - General Information on Hepatotoxicity - Hepatic failure resulting in fatalities has occurred in patients receiving valproate. These incidents usually have occurred during the ...
5.1 Hepatotoxicity
General Information on Hepatotoxicity
Hepatic failure resulting in fatalities has occurred in patients receiving valproate. These incidents usually have occurred during the first six months of treatment. Serious or fatal hepatotoxicity may be preceded by non-specific symptoms such as malaise, weakness, lethargy, facial edema, anorexia, and vomiting. In patients with epilepsy, a loss of seizure control may also occur. Patients should be monitored closely for appearance of these symptoms. Serum liver tests should be performed prior to therapy and at frequent intervals thereafter, especially during the first six months of valproate therapy. However, healthcare providers should not rely totally on serum biochemistry since these tests may not be abnormal in all instances, but should also consider the results of careful interim medical history and physical examination.
Caution should be observed when administering valproate products to patients with a prior history of hepatic disease. Patients on multiple anticonvulsants, children, those with congenital metabolic disorders, those with severe seizure disorders accompanied by mental retardation, and those with organic brain disease may be at particular risk. See below, "Patients with Known or Suspected Mitochondrial Disease."
Experience has indicated that children under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those with the aforementioned conditions. When divalproex sodium is used in this patient group, it should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. In progressively older patient groups experience in epilepsy has indicated that the incidence of fatal hepatotoxicity decreases considerably.
Patients with Known or Suspected Mitochondrial Disease
Divalproex sodium delayed-release capsules (sprinkle) are contraindicated in patients known to have mitochondrial disorders caused by POLG mutations and children under two years of age who are clinically suspected of having a mitochondrial disorder [see CONTRAINDICATIONS ( 4)] . Valproate-induced acute liver failure and liver-related deaths have been reported in patients with hereditary neurometabolic syndromes caused by mutations in the gene for mitochondrial DNA polymerase γ (POLG) (e.g., Alpers-Huttenlocher Syndrome) at a higher rate than those without these syndromes. Most of the reported cases of liver failure in patients with these syndromes have been identified in children and adolescents.
POLG-related disorders should be suspected in patients with a family history or suggestive symptoms of a POLG-related disorder, including but not limited to unexplained encephalopathy, refractory epilepsy (focal, myoclonic), status epilepticus at presentation, developmental delays, psychomotor regression, axonal sensorimotor neuropathy, myopathy cerebellar ataxia, ophthalmoplegia, or complicated migraine with occipital aura. POLG mutation testing should be performed in accordance with current clinical practice for the diagnostic evaluation of such disorders. The A467T and W748S mutations are present in approximately 2/3 of patients with autosomal recessive POLG-related disorders.
In patients over two years of age who are clinically suspected of having a hereditary mitochondrial disease, divalproex sodium delayed-release capsules (sprinkle) should only be used after other anticonvulsants have failed. This older group of patients should be closely monitored during treatment with divalproex sodium delayed-release capsules (sprinkle) for the development of acute liver injury with regular clinical assessments and serum liver test monitoring.
The drug should be discontinued immediately in the presence of significant hepatic dysfunction, suspected or apparent. In some cases, hepatic dysfunction has progressed in spite of discontinuation of drug [see BOXED WARNINGand CONTRAINDICATIONS ( 4) ].
5.2 Birth Defects
Valproate can cause fetal harm when administered to a pregnant woman. Pregnancy registry data show that maternal valproate use can cause neural tube defects and other structural abnormalities (e.g., craniofacial defects, cardiovascular malformations, hypospadias, limb malformations). The rate of congenital malformations among babies born to mothers using valproate is about four times higher than the rate among babies born to epileptic mothers using other anti-seizure monotherapies. Evidence suggests that folic acid supplementation prior to conception and during the first trimester of pregnancy decreases the risk for congenital neural tube defects in the general population.
5.3 Decreased IQ Following in utero Exposure
Valproate can cause decreased IQ scores following in uteroexposure. Published epidemiological studies have indicated that children exposed to valproate in uterohave lower cognitive test scores than children exposed in uteroto either another antiepileptic drug or to no antiepileptic drugs. The largest of these studies 1is a prospective cohort study conducted in the United States and United Kingdom that found that children with prenatal exposure to valproate (n=62) had lower IQ scores at age 6 (97 [95% C.I. 94 to 101]) than children with prenatal exposure to the other antiepileptic drug monotherapy treatments evaluated: lamotrigine (108 [95% C.I. 105 to 110]), carbamazepine (105 [95% C.I. 102 to 108]), and phenytoin (108 [95% C.I. 104 to 112]). It is not known when during pregnancy cognitive effects in valproate-exposed children occur. Because the women in this study were exposed to antiepileptic drugs throughout pregnancy, whether the risk for decreased IQ was related to a particular time period during pregnancy could not be assessed.
Although all of the available studies have methodological limitations, the weight of the evidence supports the conclusion that valproate exposure in uterocan cause decreased IQ in children.
In animal studies, offspring with prenatal exposure to valproate had malformations similar to those seen in humans and demonstrated neurobehavioral deficits [SeeUSE IN SPECIFIC POPULATIONS ( 8.1)].
Women with epilepsy who are pregnant or who plan to become pregnant should not be treated with valproate unless other treatments have failed to provide adequate symptom control or are otherwise unacceptable. In such women, the benefits of treatment with valproate during pregnancy may still outweigh the risks.
5.4 Use in Women of Childbearing Potential
Because of the risk to the fetus of decreased IQ and major congenital malformations (including neural tube defects), which may occur very early in pregnancy, valproate should not be administered to a woman of childbearing potential unless the drug is essential to the management of her medical condition. This is especially important when valproate use is considered for a condition not usually associated with permanent injury or death (e.g., migraine). Women should use effective contraception while using valproate. Women who are planning a pregnancy should be counseled regarding the relative risks and benefits of valproate use during pregnancy, and alternative therapeutic options should be considered for these patients [see BOXED WARNINGAND USE IN SPECIFIC POPULATIONS ( 8.1)].
To prevent major seizures, valproate should not be discontinued abruptly, as this can precipitate status epilepticus with resulting maternal and fetal hypoxia and threat to life.
Evidence suggests that folic acid supplementation prior to conception and during the first trimester of pregnancy decreases the risk for congenital neural tube defects in the general population. It is not known whether the risk of neural tube defects or decreased IQ in the offspring of women receiving valproate is reduced by folic acid supplementation. Dietary folic acid supplementation both prior to conception and during pregnancy should be routinely recommended for patients using valproate.
5.5 Pancreatitis
Cases of life-threatening pancreatitis have been reported in both children and adults receiving valproate. Some of the cases have been described as hemorrhagic with rapid progression from initial symptoms to death. Some cases have occurred shortly after initial use as well as after several years of use. The rate based upon the reported cases exceeds that expected in the general population and there have been cases in which pancreatitis recurred after rechallenge with valproate. In clinical trials, there were 2 cases of pancreatitis without alternative etiology in 2,416 patients, representing 1,044 patient-years experience. Patients and guardians should be warned that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis that require prompt medical evaluation. If pancreatitis is diagnosed, divalproex sodium delayed-release capsules (sprinkle) should ordinarily be discontinued. Alternative treatment for the underlying medical condition should be initiated as clinically indicated [see BOXED WARNING].
5.6 Urea Cycle Disorders
Divalproex sodium is contraindicated in patients with known urea cycle disorders (UCD). Hyperammonemic encephalopathy, sometimes fatal, has been reported following initiation of valproate therapy in patients with urea cycle disorders, a group of uncommon genetic abnormalities, particularly ornithine transcarbamylase deficiency. Prior to the initiation of divalproex sodium therapy, evaluation for UCD should be considered in the following patients: 1) those with a history of unexplained encephalopathy or coma, encephalopathy associated with a protein load, pregnancy-related or postpartum encephalopathy, unexplained mental retardation, or history of elevated plasma ammonia or glutamine; 2) those with cyclical vomiting and lethargy, episodic extreme irritability, ataxia, low BUN, or protein avoidance; 3) those with a family history of UCD or a family history of unexplained infant deaths (particularly males); 4) those with other signs or symptoms of UCD. Patients who develop symptoms of unexplained hyperammonemic encephalopathy while receiving valproate therapy should receive prompt treatment (including discontinuation of valproate therapy) and be evaluated for underlying urea cycle disorders [see CONTRAINDICATIONS ( 4) and WARNINGS AND PRECAUTIONS ( 5.10) ]
5.7 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including divalproex sodium, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to100 years) in the clinical trials analyzed.
Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
Table 1 Risk by indication for antiepileptic drugs in the pooled analysis Indication
Placebo Patients with Events Per 1,000 Patients
Drug Patients with Events Per 1,000 Patients
Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients
Risk Difference: Additional Drug Patients with Events Per 1,000 Patients
Epilepsy
1
3.4
3.5
2.4
Psychiatric
5.7
8.5
1.5
2.9
Other
1
1.8
1.9
0.9
Total
2.4
4.3
1.8
1.9
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications. Anyone considering prescribing divalproex sodium or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated. Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
5.8 Bleeding and Other Hematopoietic Disorders
Valproate is associated with dose-related thrombocytopenia. In a clinical trial of valproate as monotherapy in patients with epilepsy, 34/126 patients (27%) receiving approximately 50 mg/kg/day on average, had at least one value of platelets ≤ 75 x 10 9/L. Approximately half of these patients had treatment discontinued, with return of platelet counts to normal. In the remaining patients, platelet counts normalized with continued treatment. In this study, the probability of thrombocytopenia appeared to increase significantly at total valproate concentrations of ≥ 110 mcg/mL (females) or ≥ 135 mcg/mL (males). The therapeutic benefit which may accompany the higher doses should therefore be weighed against the possibility of a greater incidence of adverse effects. Valproate use has also been associated with decreases in other cell lines and myelodysplasia.
Because of reports of cytopenias, inhibition of the secondary phase of platelet aggregation, and abnormal coagulation parameters, (e.g., low fibrinogen, coagulation factor deficiencies, acquired von Willebrand's disease), measurements of complete blood counts and coagulation tests are recommended before initiating therapy and at periodic intervals. It is recommended that patients receiving divalproex sodium be monitored for blood counts and coagulation parameters prior to planned surgery and during pregnancy [see Use in Specific Populations ( 8.1)] . Evidence of hemorrhage, bruising, or a disorder of hemostasis/coagulation is an indication for reduction of the dosage or withdrawal of therapy.
5.9 Hyperammonemia
Hyperammonemia has been reported in association with valproate therapy and may be present despite normal liver function tests. In patients who develop unexplained lethargy and vomiting or changes in mental status, hyperammonemic encephalopathy should be considered and an ammonia level should be measured.
Hyperammonemia should also be considered in patients who present with hypothermia [see WARNINGS AND PRECAUTIONS( 5.11) ]. If ammonia is increased, valproate therapy should be discontinued. Appropriate interventions for treatment of hyperammonemia should be initiated, and such patients should undergo investigation for underlying urea cycle disorders [see CONTRAINDICATIONS ( 4) and WARNINGS AND PRECAUTIONS ( 5.6, 5.10) ]. Asymptomatic elevations of ammonia are more common and when present, require close monitoring of plasma ammonia levels. If the elevation persists, discontinuation of valproate therapy should be considered.
5.10 Hyperammonemia and Encephalopathy associated with Concomitant Topiramate Use
Concomitant administration of topiramate and valproate has been associated with hyperammonemia with or without encephalopathy in patients who have tolerated either drug alone. Clinical symptoms of hyperammonemic encephalopathy often include acute alterations in level of consciousness and/or cognitive function with lethargy or vomiting. Hypothermia can also be a manifestation of hyperammonemia [see WARNINGS AND PRECAUTIONS ( 5.11) ]. In most cases, symptoms and signs abated with discontinuation of either drug. This adverse reaction is not due to a pharmacokinetic interaction. Patients with inborn errors of metabolism or reduced hepatic mitochondrial activity may be at an increased risk for hyperammonemia with or without encephalopathy. Although not studied, an interaction of topiramate and valproate may exacerbate existing defects or unmask deficiencies in susceptible persons. In patients who develop unexplained lethargy, vomiting, or changes in mental status, hyperammonemic encephalopathy should be considered and an ammonia level should be measured. [see CONTRAINDICATIONS ( 4) and WARNINGS AND PRECAUTIONS ( 5.6, 5.9) ].
5.11 Hypothermia
Hypothermia, defined as an unintentional drop in body core temperature to < 35°C (95°F), has been reported in association with valproate therapy both in conjunction with and in the absence of hyperammonemia. This adverse reaction can also occur in patients using concomitant topiramate with valproate after starting topiramate treatment or after increasing the daily dose of topiramate [see DRUG INTERACTIONS ( 7.3) ]. Consideration should be given to stopping valproate in patients who develop hypothermia, which may be manifested by a variety of clinical abnormalities including lethargy, confusion, coma, and significant alterations in other major organ systems such as the cardiovascular and respiratory systems. Clinical management and assessment should include examination of blood ammonia levels.
5.12 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity Reactions
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as Multiorgan Hypersensitivity, has been reported in patients taking valproate. DRESS may be fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, and/or lymphadenopathy, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. Valproate should be discontinued and not be resumed if an alternative etiology for the signs or symptoms cannot be established.
5.13 Interaction with Carbapenem Antibiotics
Carbapenem antibiotics (for example, ertapenem, imipenem, meropenem; this is not a complete list) may reduce serum valproate concentrations to subtherapeutic levels, resulting in loss of seizure control. Serum valproate concentrations should be monitored frequently after initiating carbapenem therapy. Alternative antibacterial or anticonvulsant therapy should be considered if serum valproate concentrations drop significantly or seizure control deteriorates [see DRUG INTERACTIONS ( 7.1) ].
5.14 Somnolence in the Elderly
In a doubleblind, multicenter trial of valproate in elderly patients with dementia (mean age = 83 years), doses were increased by 125 mg/day to a target dose of 20 mg/kg/day .A significantly higher proportion of valproate patients had somnolence compared to placebo, and although not statistically significant, there was a higher proportion of patients with dehydration. Discontinuations for somnolence were also significantly higher than with placebo .In some patients with somnolence (approximately one-half), there was associated reduced nutritional intake and weight loss .There was a trend for the patients who experienced these events to have a lower baseline albumin concentration, lower valproate clearance, and a higher BUN. In elderly patients, dosage should be increased more slowly and with regular monitoring for fluid and nutritional intake, dehydration, somnolence, and other adverse reactions. Dose reductions or discontinuation of valproate should be considered in patients with decreased food or fluid intake and in patients with excessive somnolence [see DOSAGE AND ADMINISTRATION ( 2.2) ].
5.15 Monitoring: Drug Plasma Concentration
Since valproate may interact with concurrently administered drugs which are capable of enzyme induction, periodic plasma concentration determinations of valproate and concomitant drugs are recommended during the early course of therapy [see DRUG INTERACTIONS ( 7) ].
5.16 Effect on Ketone and Thyroid Function Tests
Valproate is partially eliminated in the urine as a keto-metabolite which may lead to a false interpretation of the urine ketone test.
There have been reports of altered thyroid function tests associated with valproate. The clinical significance of these is unknown.
5.17 Effect on HIV and CMV Viruses Replication
There are in vitrostudies that suggest valproate stimulates the replication of the HIV and CMV viruses under certain experimental conditions. The clinical consequence, if any, is not known. Additionally, the relevance of these in vitrofindings is uncertain for patients receiving maximally suppressive antiretroviral therapy. Nevertheless, these data should be borne in mind when interpreting the results from regular monitoring of the viral load in HIV infected patients receiving valproate or when following CMV infected patients clinically.
Close5.18 Medication Residue in the Stool
There have been rare reports of medication residue in the stool. Some patients have had anatomic (including ileostomy or colostomy) or functional gastrointestinal disorders with shortened GI transit times. In some reports, medication residues have occurred in the context of diarrhea. It is recommended that plasma valproate levels be checked in patients who experience medication residue in the stool, and patients' clinical condition should be monitored. If clinically indicated, alternative treatment may be considered.
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: Hepatic failure - [see Warnings and Precautions ( 5.1)] Birth defects - [see ...
The following serious adverse reactions are described below and elsewhere in the labeling:
- Hepatic failure [see Warnings and Precautions ( 5.1)]
- Birth defects [see Warnings and Precautions ( 5.2)]
- Decreased IQ following in uteroexposure [see Warnings and Precautions ( 5.3)]
- Pancreatitis [see Warnings and Precautions ( 5.5)]
- Hyperammonemic encephalopathy [see Warnings and Precautions ( 5.6, 5.9, 5.10)]
- Suicidal behavior and ideation [see Warnings and Precautions ( 5.7)]
- Bleeding and other hematopoietic disorders [see Warnings and Precautions ( 5.8)]
- Hypothermia [see Warnings and Precautions ( 5.11)]
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan hypersensitivity reactions [see Warnings and Precautions ( 5.12)]
- Somnolence in the elderly [see Warnings and Precautions ( 5.14)]
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
6.1 Epilepsy
Based on a placebo-controlled trial of adjunctive therapy for treatment of complex partial seizures, divalproex sodium delayed-release tablets was generally well tolerated with most adverse reactions rated as mild to moderate in severity. Intolerance was the primary reason for discontinuation in the divalproex sodium delayed-release tablets-treated patients (6%), compared to 1% of placebo-treated patients.
In a long term (12 month) safety study in pediatric patients (n=169) between the ages of 3 and 10 years old, no clinically meaningful differences in the adverse event profile were observed when compared to adults.
Table 2 lists treatment-emergent adverse reactions which were reported by ≥ 5% of divalproex sodium delayed-release tablets-treated patients and for which the incidence was greater than in the placebo group, in the placebo-controlled trial of adjunctive therapy for treatment of complex partial seizures. Since patients were also treated with other antiepilepsy drugs, it is not possible, in most cases, to determine whether the following adverse reactions can be ascribed to divalproex sodium delayed-release tablets alone, or the combination of divalproex sodium delayed-release tablets and other antiepilepsy drugs.
Table 2 Adverse Reactions Reported by > 5% of Patients Treated with Valproate During Placebo-Controlled Trial of Adjunctive Therapy for Complex Partial Seizures Body System/Event
Divalproex Sodium Delayed-release Tablets (%)
Placebo (%)
(n = 77)
(n = 70)
Body as a Whole
Headache
31
21
Asthenia
27
7
Fever
6
4
Gastrointestinal System
Nausea
48
14
Vomiting
27
7
Abdominal Pain
23
6
Diarrhea
13
6
Anorexia
12
0
Dyspepsia
8
4
Constipation
5
1
Nervous System
Somnolence
27
11
Tremor
25
6
Dizziness
25
13
Diplopia
16
9
Amblyopia/Blurred Vision
12
9
Ataxia
8
1
Nystagmus
8
1
Emotional Lability
6
4
Thinking Abnormal
6
0
Amnesia
5
1
Respiratory System
Flu Syndrome
12
9
Infection
12
6
Bronchitis
5
1
Rhinitis
5
4
Other
Alopecia
6
1
Weight Loss
6
0
Table 3 lists treatment-emergent adverse reactions which were reported by ≥ 5% of patients in the high dose valproate group, and for which the incidence was greater than in the low dose group, in a controlled trial of divalproex sodium delayed-release tablets monotherapy treatment of complex partial seizures. Since patients were being titrated off another antiepilepsy drug during the first portion of the trial, it is not possible, in many cases, to determine whether the following adverse reactions can be ascribed to divalproex sodium delayed-release tablets alone, or the combination of valproate and other antiepilepsy drugs.
Table 3 Adverse Reactions Reported by > 5% of Patients in the High Dose Group in the Controlled Trial of Valproate Monotherapy for Complex Partial Seizures 1 1Headache was the only adverse event that occurred in ≥ 5% of patients in the high dose group and at an equal or greater incidence in the low dose group.
Body System/Event
High Dose (%)
Low Dose (%)
(n = 131)
(n = 134)
Body as a Whole
Asthenia
21
10
Digestive System
Nausea
34
26
Diarrhea
23
19
Vomiting
23
15
Abdominal Pain
12
9
Anorexia
11
4
Dyspepsia
11
10
Hemic/Lymphatic System
Thrombocytopenia
24
1
Ecchymosis
5
4
Metabolic/Nutritional
Weight Gain
9
4
Peripheral Edema
8
3
Nervous System
Tremor
57
19
Somnolence
30
18
Dizziness
18
13
Insomnia
15
9
Nervousness
11
7
Amnesia
7
4
Nystagmus
7
1
Depression
5
4
Respiratory System
Infection
20
13
Pharyngitis
8
2
Dyspnea
5
1
Skin and Appendages
Alopecia
24
13
Special Senses
Amblyopia/Blurred Vision
8
4
Tinnitus
7
1
The following additional adverse reactions were reported by greater than 1% but less than 5% of the 358 patients treated with valproate in the controlled trials of complex partial seizures:
Body as a Whole
Back pain, chest pain, malaise.
Cardiovascular System
Tachycardia, hypertension, palpitation.
Digestive System
Increased appetite, flatulence, hematemesis, eructation, pancreatitis, periodontal abscess.
Hemic and Lymphatic System
Petechia.
Metabolic and Nutritional Disorders
SGOT increased, SGPT increased.
Musculoskeletal System
Myalgia, twitching, arthralgia, leg cramps, myasthenia.
Nervous System
Anxiety, confusion, abnormal gait, paresthesia, hypertonia, incoordination, abnormal dreams, personality disorder.
Respiratory System
Sinusitis, cough increased, pneumonia, epistaxis.
Skin and Appendages
Rash, pruritus, dry skin.
Special Senses
Taste perversion, abnormal vision, deafness, otitis media.
Urogenital System
Urinary incontinence, vaginitis, dysmenorrhea, amenorrhea, urinary frequency.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of divalproex sodium. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Dermatologic
Hair texture changes, hair color changes, photosensitivity, erythema multiforme, toxic epidermal necrolysis, nail and nail bed disorders, and Stevens-Johnson syndrome.
Psychiatric
Emotional upset, psychosis, aggression, psychomotor hyperactivity, hostility, disturbance in attention, learning disorder, and behavioral deterioration.
Neurologic
Paradoxical convulsion, parkinsonism
There have been several reports of acute or subacute cognitive decline and behavioral changes (apathy or irritability) with cerebral pseudoatrophy on imaging associated with valproate therapy; both the cognitive/behavioral changes and cerebral pseudoatrophy reversed partially or fully after valproate discontinuation.
There have been reports of acute or subacute encephalopathy in the absence of elevated ammonia levels, elevated valproate levels, or neuroimaging changes. The encephalopathy reversed partially or fully after valproate discontinuation.
Musculoskeletal
Fractures, decreased bone mineral density, osteopenia, osteoporosis, and weakness.
Hematologic
Relative lymphocytosis, macrocytosis, leukopenia, anemia including macrocytic with or without folate deficiency, bone marrow suppression, pancytopenia, aplastic anemia, agranulocytosis, and acute intermittent porphyria.
Endocrine
Irregular menses, secondary amenorrhea, hyperandrogenism, hirsutism, elevated testosterone level, breast enlargement, galactorrhea, parotid gland swelling, polycystic ovary disease, decreased carnitine concentrations, hyponatremia, hyperglycinemia, and inappropriate ADH secretion.
There have been rare reports of Fanconi's syndrome occurring chiefly in children.
Metabolism and nutrition
Weight gain.
Reproductive
Aspermia, azoospermia, decreased sperm count, decreased spermatozoa motility, male infertility, and abnormal spermatozoa morphology.
Genitourinary
Enuresis and urinary tract infection.
Special Senses
Hearing loss.
Other
Allergic reaction, anaphylaxis, developmental delay, bone pain, bradycardia, and cutaneous vasculitis.
-
7 DRUG INTERACTIONS7.1 Effects of Coadministered Drugs onValproate Clearance - Drugs that affect the level of expression of hepatic enzymes, particularly those that elevate levels of glucuronosyltransferases (such ...
7.1 Effects of Coadministered Drugs onValproate Clearance
Drugs that affect the level of expression of hepatic enzymes, particularly those that elevate levels of glucuronosyltransferases (such as ritonavir), may increase the clearance of valproate. For example, phenytoin, carbamazepine, and phenobarbital (or primidone) can double the clearance of valproate. Thus, patients on monotherapy will generally have longer half-lives and higher concentrations than patients receiving polytherapy with antiepilepsy drugs.
In contrast, drugs that are inhibitors of cytochrome P450 isozymes, e.g., antidepressants, may be expected to have little effect on valproate clearance because cytochrome P450 microsomal mediated oxidation is a relatively minor secondary metabolic pathway compared to glucuronidation and beta-oxidation.
Because of these changes in valproate clearance, monitoring of valproate and concomitant drug concentrations should be increased whenever enzyme inducing drugs are introduced or withdrawn.
The following list provides information about the potential for an influence of several commonly prescribed medications on valproate pharmacokinetics. The list is not exhaustive nor could it be, since new interactions are continuously being reported.
Drugs for which a potentially important interaction has been observed
Aspirin
A study involving the coadministration of aspirin at antipyretic doses (11 to 16 mg/kg) with valproate to pediatric patients (n=6) revealed a decrease in protein binding and an inhibition of metabolism of valproate. Valproate free fraction was increased 4-fold in the presence of aspirin compared to valproate alone. The β-oxidation pathway consisting of 2-E-valproic acid, 3-OH-valproic acid, and 3-keto valproic acid was decreased from 25% of total metabolites excreted on valproate alone to 8.3% in the presence of aspirin. Caution should be observed if valproate and aspirin are to be coadministered.
Carbapenem Antibiotics
A clinically significant reduction in serum valproic acid concentration has been reported in patients receiving carbapenem antibiotics (for example, ertapenem, imipenem, meropenem; this is not a complete list) and may result in loss of seizure control. The mechanism of this interaction is not well understood. Serum valproic acid concentrations should be monitored frequently after initiating carbapenem therapy. Alternative antibacterial or anticonvulsant therapy should be considered if serum valproic acid concentrations drop significantly or seizure control deteriorates [see WARNINGS AND PRECAUTIONS ( 5.13) ].
Estrogen-Containing Hormonal Contraceptives
Estrogen-containing hormonal contraceptives may increase the clearance of valproate, which may result in decreased concentration of valproate and potentially increased seizure frequency. Prescribers should monitor serum valproate concentrations and clinical response when adding or discontinuing estrogen containing products.
Felbamate
A study involving the coadministration of 1,200 mg/day of felbamate with valproate to patients with epilepsy (n=10) revealed an increase in mean valproate peak concentration by 35% (from 86 to 115 mcg/mL) compared to valproate alone. Increasing the felbamate dose to 2,400 mg/day increased the mean valproate peak concentration to 133 mcg/mL (another 16% increase). A decrease in valproate dosage may be necessary when felbamate therapy is initiated.
Rifampin
A study involving the administration of a single dose of valproate (7 mg/kg) 36 hours after 5 nights of daily dosing with rifampin (600 mg) revealed a 40% increase in the oral clearance of valproate. Valproate dosage adjustment may be necessary when it is coadministered with rifampin.
Drugs for which either no interaction or a likely clinically unimportant interaction has been observed
Antacids
A study involving the coadministration of valproate 500 mg with commonly administered antacids (Maalox, Trisogel, and Titralac - 160 mEq doses) did not reveal any effect on the extent of absorption of valproate.
Chlorpromazine
A study involving the administration of 100 to 300 mg/day of chlorpromazine to schizophrenic patients already receiving valproate (200 mg BID) revealed a 15% increase in trough plasma levels of valproate.
Haloperidol
A study involving the administration of 6 to 10 mg/day of haloperidol to schizophrenic patients already receiving valproate (200 mg BID) revealed no significant changes in valproate trough plasma levels.
Cimetidine and Ranitidine
Cimetidine and ranitidine do not affect the clearance of valproate.
7.2 Effects of Valproate on Other Drugs
Valproate has been found to be a weak inhibitor of some P450 isozymes, epoxide hydrase, and glucuronosyltransferases. The following list provides information about the potential for an influence of valproate coadministration on the pharmacokinetics or pharmacodynamics of several commonly prescribed medications. The list is not exhaustive, since new interactions are continuously being reported.
Drugs for which a potentially important valproate interaction has been observed
Amitriptyline/Nortriptyline
Administration of a single oral 50 mg dose of amitriptyline to 15 normal volunteers (10 males and 5 females) who received valproate (500 mg BID) resulted in a 21% decrease in plasma clearance of amitriptyline and a 34% decrease in the net clearance of nortriptyline. Rare postmarketing reports of concurrent use of valproate and amitriptyline resulting in an increased amitriptyline level have been received. Concurrent use of valproate and amitriptyline has rarely been associated with toxicity. Monitoring of amitriptyline levels should be considered for patients taking valproate concomitantly with amitriptyline. Consideration should be given to lowering the dose of amitriptyline/nortriptyline in the presence of valproate.
Carbamazepine/carbamazepine-10,11-Epoxide
Serum levels of carbamazepine (CBZ) decreased 17% while that of carbamazepine-10,11-epoxide (CBZ-E) increased by 45% upon coadministration of valproate and CBZ to epileptic patients.
Clonazepam
The concomitant use of valproate and clonazepam may induce absence status in patients with a history of absence type seizures.
Diazepam
Valproate displaces diazepam from its plasma albumin binding sites and inhibits its metabolism. Coadministration of valproate (1,500 mg daily) increased the free fraction of diazepam (10 mg) by 90% in healthy volunteers (n=6). Plasma clearance and volume of distribution for free diazepam were reduced by 25% and 20%, respectively, in the presence of valproate. The elimination half-life of diazepam remained unchanged upon addition of valproate.
Ethosuximide
Valproate inhibits the metabolism of ethosuximide. Administration of a single ethosuximide dose of 500 mg with valproate (800 to 1,600 mg/day) to healthy volunteers (n=6) was accompanied by a 25% increase in elimination half-life of ethosuximide and a 15% decrease in its total clearance as compared to ethosuximide alone. Patients receiving valproate and ethosuximide, especially along with other anticonvulsants, should be monitored for alterations in serum concentrations of both drugs.
Lamotrigine
In a steady-state study involving 10 healthy volunteers, the elimination half-life of lamotrigine increased from 26 to 70 hours with valproate coadministration (a 165% increase). The dose of lamotrigine should be reduced when coadministered with valproate. Serious skin reactions (such as Stevens-Johnson syndrome and toxic epidermal necrolysis) have been reported with concomitant lamotrigine and valproate administration. See lamotrigine package insert for details on lamotrigine dosing with concomitant valproate administration.
Phenobarbital
Valproate was found to inhibit the metabolism of phenobarbital. Coadministration of valproate (250 mg BID for 14 days) with phenobarbital to normal subjects (n=6) resulted in a 50% increase in half-life and a 30% decrease in plasma clearance of phenobarbital (60 mg single-dose). The fraction of phenobarbital dose excreted unchanged increased by 50% in presence of valproate.
There is evidence for severe CNS depression, with or without significant elevations of barbiturate or valproate serum concentrations. All patients receiving concomitant barbiturate therapy should be closely monitored for neurological toxicity. Serum barbiturate concentrations should be obtained, if possible, and the barbiturate dosage decreased, if appropriate. Primidone, which is metabolized to a barbiturate, may be involved in a similar interaction with valproate.
Phenytoin
Valproate displaces phenytoin from its plasma albumin binding sites and inhibits its hepatic metabolism. Coadministration of valproate (400 mg TID) with phenytoin (250 mg) in normal volunteers (n=7) was associated with a 60% increase in the free fraction of phenytoin. Total plasma clearance and apparent volume of distribution of phenytoin increased 30% in the presence of valproate. Both the clearance and apparent volume of distribution of free phenytoin were reduced by 25%. In patients with epilepsy, there have been reports of breakthrough seizures occurring with the combination of valproate and phenytoin. The dosage of phenytoin should be adjusted as required by the clinical situation.
Propofol
The concomitant use of valproate and propofol may lead to increased blood levels of propofol. Reduce the dose of propofol when co-administering with valproate. Monitor patients closely for signs of increased sedation or cardiorespiratory depression.
Rufinamide
Based on a population pharmacokinetic analysis, rufinamide clearance was decreased by valproate. Rufinamide concentrations were increased by <16% to 70%, dependent on concentration of valproate (with the larger increases being seen in pediatric patients at high doses or concentrations of valproate). Patients stabilized on rufinamide before being prescribed valproate should begin valproate therapy at a low dose, and titrate to a clinically effective dose [see Dosage and Administration ( 2.3)] . Similarly, patients on valproate should begin at a rufinamide dose lower than 10 mg/kg per day (pediatric patients) or 400 mg per day (adults).
Tolbutamide
From in vitroexperiments, the unbound fraction of tolbutamide was increased from 20% to 50% when added to plasma samples taken from patients treated with valproate. The clinical relevance of this displacement is unknown.
Warfarin
In an in vitrostudy, valproate increased the unbound fraction of warfarin by up to 32.6%. The therapeutic relevance of this is unknown; however, coagulation tests should be monitored if valproate therapy is instituted in patients taking anticoagulants.
Zidovudine
In six patients who were seropositive for HIV, the clearance of zidovudine (100 mg q8h) was decreased by 38% after administration of valproate (250 or 500 mg q8h); the half-life of zidovudine was unaffected.
Drugs for which either no interaction or a likely clinically unimportant interaction has been observed
Acetaminophen
Valproate had no effect on any of the pharmacokinetic parameters of acetaminophen when it was concurrently administered to three epileptic patients.
Clozapine
In psychotic patients (n=11), no interaction was observed when valproate was coadministered with clozapine.
Lithium
Coadministration of valproate (500 mg BID) and lithium carbonate (300 mg TID) to normal male volunteers (n=16) had no effect on the steady-state kinetics of lithium.
Lorazepam
Concomitant administration of valproate (500 mg BID) and lorazepam (1 mg BID) in normal male volunteers (n=9) was accompanied by a 17% decrease in the plasma clearance of lorazepam.
Olanzapine
No dose adjustment for olanzapine is necessary when olanzapine is administered concomitantly with valproate. Coadministration of valproate (500 mg BID) and olanzapine (5 mg) to healthy adults (n=10) caused 15% reduction in C maxand 35% reduction in AUC of olanzapine.
Oral Contraceptive Steroids
Administration of a single-dose of ethinyloestradiol (50 mcg)/levonorgestrel (250 mcg) to 6 women on valproate (200 mg BID) therapy for 2 months did not reveal any pharmacokinetic interaction.
Close7.3 Topiramate
Concomitant administration of valproate and topiramate has been associated with hyperammonemia with and without encephalopathy [see CONTRAINDICATIONS ( 4) and WARNINGS AND PRECAUTIONS ( 5.6, 5.9, 5.10) ].
Concomitant administration of topiramate with valproate has also been associated with hypothermia in patients who have tolerated either drug alone. It may be prudent to examine blood ammonia levels in patients in whom the onset of hypothermia has been reported [see WARNINGS AND PRECAUTIONS ( 5.9, 5.11) ].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category Dfor epilepsy - [seeWARNINGS AND PRECAUTIONS ( 5.2, 5.3)]. Pregnancy Registry - To collect information on the effects of - in ...
8.1 Pregnancy
Pregnancy Category Dfor epilepsy [seeWARNINGS AND PRECAUTIONS ( 5.2, 5.3)].
Pregnancy Registry
To collect information on the effects of in uteroexposure to divalproex sodium, physicians should encourage pregnant patients taking divalproex sodium to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling toll free 1-888-233-2334, and must be done by the patients themselves.
Information on the registry can be found at the website, http://www.aedpregnancyregistry.org/.
Fetal Risk Summary
All pregnancies have a background risk of birth defects (about 3%), pregnancy loss (about 15%), or other adverse outcomes regardless of drug exposure. Maternal valproate use during pregnancy for any indication increases the risk of congenital malformations, particularly neural tube defects, but also malformations involving other body systems (e.g., craniofacial defects, cardiovascular malformations, hypospadias, limb malformations). The risk of major structural abnormalities is greatest during the first trimester; however, other serious developmental effects can occur with valproate use throughout pregnancy. The rate of congenital malformations among babies born to epileptic mothers who used valproate during pregnancy has been shown to be about four times higher than the rate among babies born to epileptic mothers who used other anti-seizure monotherapies [see WARNINGS AND PRECAUTIONS ( 5.3) ].
Several published epidemiological studies have indicated that children exposed to valproate in uterohave lower IQ scores than children exposed to either another antiepileptic drug in uteroor to no antiepileptic drugs in utero[see WARNINGS AND PRECAUTIONS ( 5.3) ].
An observational study has suggested that exposure to valproate products during pregnancy may increase the risk of autism spectrum disorders. In this study, children born to mothers who had used valproate products during pregnancy had 2.9 times the risk (95% confidence interval [CI]: 1.7 to 4.9) of developing autism spectrum disorders compared to children born to mothers not exposed to valproate products during pregnancy. The absolute risks for autism spectrum disorders were 4.4% (95% CI: 2.6% to 7.5%) in valproate-exposed children and 1.5% (95% CI: 1.5% to 1.6%) in children not exposed to valproate products. Because the study was observational in nature, conclusions regarding a causal association between in uterovalproate exposure and an increased risk of autism spectrum disorder cannot be considered definitive.
In animal studies, offspring with prenatal exposure to valproate had structural malformations similar to those seen in humans and demonstrated neuro behavioral deficits.
Clinical Considerations
- Neural tube defects are the congenital malformation most strongly associated with maternal valproate use. The risk of spina bifida following in uterovalproate exposure is generally estimated as 1 to 2%, compared to an estimated general population risk for spina bifida of about 0.06 to 0.07% (6 to 7 in 10,000 births).
- Valproate can cause decreased IQ scores in children whose mothers were treated with valproate during pregnancy.
- Because of the risks of decreased IQ, neural tube defects, and other fetal adverse events, which may occur very early in pregnancy:
- Valproate should not be administered to a woman of childbearing potential unless the drug is essential to the management of her medical condition. This is especially important when valproate use is considered for a condition not usually associated with permanent injury or death (e.g., migraine).
- Divalproex sodium delayed-release capsules (sprinkle) should not be used to treat women with epilepsy who are pregnant or who plan to become pregnant unless other treatments have failed to provide adequate symptom control or are otherwise unacceptable. In such women, the benefits of treatment with valproate during pregnancy may still outweigh the risks. When treating a pregnant woman or a woman of childbearing potential, carefully consider both the potential risks and benefits of treatment and provide appropriate counseling.
- To prevent major seizures, women with epilepsy should not discontinue valproate abruptly, as this can precipitate status epilepticus with resulting maternal and fetal hypoxia and threat to life. Even minor seizures may pose some hazard to the developing embryo or fetus. However, discontinuation of the drug may be considered prior to and during pregnancy in individual cases if the seizure disorder severity and frequency do not pose a serious threat to the patient.
- Available prenatal diagnostic testing to detect neural tube and other defects should be offered to pregnant women using valproate.
- Evidence suggests that folic acid supplementation prior to conception and during the first trimester of pregnancy decreases the risk for congenital neural tube defects in the general population. It is not known whether the risk of neural tube defects or decreased IQ in the offspring of women receiving valproate is reduced by folic acid supplementation. Dietary folic acid supplementation both prior to conception and during pregnancy should be routinely recommended for patients using valproate.
- Pregnant women taking valproate may develop clotting abnormalities including thrombocytopenia, hypofibrinogenemia, and/or decrease in other coagulation factors, which may result in hemorrhagic complications in the neonate including death [see Warnings and Precautions ( 5.8)] . If valproate is used in pregnancy, the clotting parameters should be monitored carefully in the mother. If abnormal in the mother, then these parameters should also be monitored in the neonate.
- Patients taking valproate may develop hepatic failure [see Boxed Warningand Warnings and Precautions ( 5.1)] . Fatal cases of hepatic failure in infants exposed to valproate in uterohave also been reported following maternal use of valproate during pregnancy.
- Hypoglycemia has been reported in neonates whose mothers have taken valproate during pregnancy.
Human
There is an extensive body of evidence demonstrating that exposure to valproate in uteroincreases the risk of neural tube defects and other structural abnormalities. Based on published data from the CDC's National Birth Defects Prevention Network, the risk of spina bifida in the general population is about 0.06 to 0.07%. The risk of spina bifida following in uterovalproate exposure has been estimated to be approximately 1 to 2%.
The NAAED Pregnancy Registry has reported a major malformation rate of 9 to 11% in the offspring of women exposed to an average of 1,000 mg/day of valproate monotherapy during pregnancy. These data show up to a five-fold increased risk for any major malformation following valproate exposure in uterocompared to the risk following exposure in uteroto other antiepileptic drugs taken in monotherapy. The major congenital malformations included cases of neural tube defects, cardiovascular malformations, craniofacial defects (e.g., oral clefts, craniosynostosis), hypospadias, limb malformations (e.g., clubfoot, polydactyly), and malformations of varying severity involving other body systems.
Published epidemiological studies have indicated that children exposed to valproate in uterohave lower IQ scores than children exposed to either another antiepileptic drug in uteroor to no antiepileptic drugs in utero. The largest of these studies is a prospective cohort study conducted in the United States and United Kingdom that found that children with prenatal exposure to valproate (n=62) had lower IQ scores at age 6 (97 [95% C.I. 94 to 101]) than children with prenatal exposure to the other anti-epileptic drug monotherapy treatments evaluated: lamotrigine (108 [95% C.I. 105 to110]), carbamazepine (105 [95% C.I. 102 to 108]) and phenytoin (108 [95% C.I. 104 to 112]). It is not known when during pregnancy cognitive effects in valproate-exposed children occur. Because the women in this study were exposed to antiepileptic drugs throughout pregnancy, whether the risk for decreased IQ was related to a particular time period during pregnancy could not be assessed.
Although all of the available studies have methodological limitations, the weight of the evidence supports a causal association between valproate exposure in uteroand subsequent adverse effects on cognitive development.
There are published case reports of fatal hepatic failure in offspring of women who used valproate during pregnancy.
Animal
In developmental toxicity studies conducted in mice, rats, rabbits, and monkeys, increased rates of fetal structural abnormalities, intrauterine growth retardation, and embryo-fetal death occurred following treatment of pregnant animals with valproate during organogenesis at clinically relevant doses (calculated on a body surface area basis). Valproate induced malformations of multiple organ systems, including skeletal, cardiac, and urogenital defects. In mice, in addition to other malformations, fetal neural tube defects have been reported following valproate administration during critical periods of organogenesis, and the teratogenic response correlated with peak maternal drug levels. Behavioral abnormalities (including cognitive, locomotor, and social interaction deficits) and brain histopathological changes have also been reported in mice and rat offspring exposed prenatally to clinically relevant doses of valproate.
8.3 Nursing Mothers
Valproate is excreted in human milk. Caution should be exercised when valproate is administered to a nursing woman.
8.4 Pediatric Use
Experience has indicated that pediatric patients under the age of two years are at a considerably increased risk of developing fatal hepatotoxicity, especially those with the aforementioned conditions [see BOXED WARNING, WARNING AND PRECAUTIONS ( 5.1) ]. When divalproex sodium is used in this patient group, it should be used with extreme caution and as a sole agent. The benefits of therapy should be weighed against the risks. Above the age of 2 years, experience in epilepsy has indicated that the incidence of fatal hepatotoxicity decreases considerably in progressively older patient groups.
Younger children, especially those receiving enzyme inducing drugs, will require larger maintenance doses to attain targeted total and unbound valproate concentrations. Pediatric patients (i.e., between 3 months and 10 years) have 50% higher clearances expressed on weight (i.e., mL/min/kg) than do adults. Over the age of 10 years, children have pharmacokinetic parameters that approximate those of adults.
The variability in free fraction limits the clinical usefulness of monitoring total serum valproic acid concentrations. Interpretation of valproic acid concentrations in children should include consideration of factors that affect hepatic metabolism and protein binding.
Pediatric Clinical Trials
Divalproex sodium was studied in seven pediatric clinical trials.
Two of the pediatric studies were doubleblinded placebo-controlled trials to evaluate the efficacy of divalproex sodium extended-release tablets for the indications of mania (150 patients aged 10 to 17 years, 76 of whom were on divalproex sodium extended-release tablets) and migraine (304 patients aged 12 to 17 years, 231 of whom were on divalproex sodium extended-release tablets). Efficacy was not established for either the treatment of migraine or the treatment of mania. The most common drug-related adverse reactions (reported > 5% and twice the rate of placebo) reported in the controlled pediatric mania study were nausea, upper abdominal pain, somnolence, increased ammonia, gastritis and rash.
The remaining five trials were long term safety studies. Two six month pediatric studies were conducted to evaluate the long-term safety of divalproex sodium extended-release tablets for the indication of mania (292 patients aged 10 to 17 years). Two twelve month pediatric studies were conducted to evaluate the long-term safety of divalproex sodium extended-release tablets for the indication of migraine (353 patients aged 12 to 17 years). One twelve month study was conducted to evaluate the safety of divalproex sodium delayed-release capsules (sprinkle) in the indication of partial seizures (169 patients aged 3 to 10 years).
In these seven clinical trials, the safety and tolerability of divalproex sodium in pediatric patients were shown to be comparable to those in adults [see ADVERSE REACTIONS ( 6) ].
Juvenile Animal Toxicology
In studies of valproate in immature animals, toxic effects not observed in adult animals included retinal dysplasia in rats treated during the neonatal period (from postnatal day 4) and nephrotoxicity in rats treated during the neonatal and juvenile (from postnatal day 14) periods. The no-effect dose for these findings was less than the maximum recommended human dose on a mg/m 2basis.
8.5 Geriatric Use
No patients above the age of 65 years were enrolled in doubleblind prospective clinical trials of mania associated with bipolar illness. In a case review study of 583 patients, 72 patients (12%) were greater than 65 years of age. A higher percentage of patients above 65 years of age reported accidental injury, infection, pain, somnolence, and tremor.
Discontinuation of valproate was occasionally associated with the latter two events. It is not clear whether these events indicate additional risk or whether they result from preexisting medical illness and concomitant medication use among these patients.
A study of elderly patients with dementia revealed drug related somnolence and discontinuation for somnolence [see WARNINGS AND PRECAUTIONS ( 5.14) ]. The starting dose should be reduced in these patients, and dosage reductions or discontinuation should be considered in patients with excessive somnolence [see DOSAGE AND ADMINISTRATION ( 2.2) ].
The capacity of elderly patients (age range: 68 to 89 years) to eliminate valproate has been shown to be reduced compared to younger adults (age range: 22 to 26 years) [see CLINICAL PHARMACOLOGY ( 12.3) ].
Close8.6 Effect of Disease
Liver disease impairs the capacity to eliminate valproate [see Boxed Warning, Contraindications ( 4), Warnings and Precautions ( 5.1), and Clinical Pharmacology ( 12.3)] .
-
10 OVERDOSAGEOverdosage with valproate may result in somnolence, heart block, deep coma and hypernatremia. Fatalities have been reported; however patients have recovered from valproate levels as high as ...Close
Overdosage with valproate may result in somnolence, heart block, deep coma and hypernatremia. Fatalities have been reported; however patients have recovered from valproate levels as high as 2120 mcg/mL.
In overdose situations, the fraction of drug not bound to protein is high and hemodialysis or tandem hemodialysis plus hemoperfusion may result in significant removal of drug. The benefit of gastric lavage or emesis will vary with the time since ingestion. General supportive measures should be applied with particular attention to the maintenance of adequate urinary output.
Naloxone has been reported to reverse the CNS depressant effects of valproate over dosage. Because naloxone could theoretically also reverse the antiepileptic effects of valproate, it should be used with caution in patients with epilepsy.
-
11 DESCRIPTIONDivalproex sodium is a stable co-ordination compound comprised of sodium valproate and valproic acid in a 1:1 molar relationship. Chemically it is designated as sodium hydrogen ...Close
Divalproex sodium is a stable co-ordination compound comprised of sodium valproate and valproic acid in a 1:1 molar relationship. Chemically it is designated as sodium hydrogen bis(2-propylpentanoate). Divalproex sodium has the following structure:
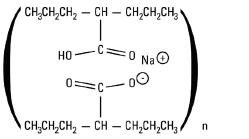
Divalproex sodium, USP occurs as a white powder with a characteristic odor.
Each divalproex sodium delayed-release capsules, USP (sprinkle) intended for oral administration contains divalproex sodium equivalent to 125 mg of valproic acid. In addition, each capsule contains the following inactive ingredients: colloidal silicon dioxide, FD &C blue # 1, gelatin, hypromellose, methacrylic acid copolymer dispersion, microcrystalline cellulose spheres, sodium lauryl sulfate, talc, titanium dioxide and triethyl citrate. Each capsule is printed with black pharmaceutical ink which contains: ammonia solution, butyl alcohol, dehydrated alcohol, ferrosoferric oxide, isopropyl alcohol, propylene glycol, potassium hydroxide, purified water and shellac.
The Product meets USP Dissolution Test 4.
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Divalproex sodium dissociates to the valproate ion in the gastrointestinal tract. The mechanisms by which valproate exerts its therapeutic effects have not been ...
12.1 Mechanism of Action
Divalproex sodium dissociates to the valproate ion in the gastrointestinal tract. The mechanisms by which valproate exerts its therapeutic effects have not been established. It has been suggested that its activity in epilepsy is related to increased brain concentrations of gamma-aminobutyric acid (GABA).
12.2 Pharmacodynamics
The relationship between plasma concentration and clinical response is not well documented. One contributing factor is the nonlinear, concentration dependent protein binding of valproate which affects the clearance of the drug. Thus, monitoring of total serum valproate may not provide a reliable index of the bioactive valproate species.
For example, because the plasma protein binding of valproate is concentration dependent, the free fraction increases from approximately 10% at 40 mcg/mL to 18.5% at 130 mcg/mL. Higher than expected free fractions occur in the elderly, in hyperlipidemic patients, and in patients with hepatic and renal diseases.
Epilepsy
The therapeutic range in epilepsy is commonly considered to be 50 to 100 mcg/mL of total valproate, although some patients may be controlled with lower or higher plasma concentrations.
Close12.3 Pharmacokinetics
Although the rate of valproate ion absorption may vary with the formulation administered (liquid, solid, or sprinkle), conditions of use (e.g., fasting or postprandial) and the method of administration (e.g., whether the contents of the capsule are sprinkled on food or the capsule is taken intact), these differences should be of minor clinical importance under the steady state conditions achieved in chronic use in the treatment of epilepsy.
However, it is possible that differences among the various valproate products in T maxand C maxcould be important upon initiation of treatment. For example, in single dose studies, the effect of feeding had a greater influence on the rate of absorption of the tablet (increase in T maxfrom 4 to 8 hours) than on the absorption of the sprinkle capsules (increase in T maxfrom 3.3 to 4.8 hours).
While the absorption rate from the G.I. tract and fluctuation in valproate plasma concentrations vary with dosing regimen and formulation, the efficacy of valproate as an anticonvulsant in chronic use is unlikely to be affected. Experience employing dosing regimens from once-a-day to four-times-a-day, as well as studies in primate epilepsy models involving constant rate infusion, indicate that total daily systemic bioavailability (extent of absorption) is the primary determinant of seizure control and that differences in the ratios of plasma peak to trough concentrations between valproate formulations are inconsequential from a practical clinical standpoint.
Coadministration of oral valproate products with food should cause no clinical problems in the management of patients with epilepsy [see Dosage and Administration ( 2.1)] .
Distribution
Protein Binding
The plasma protein binding of valproate is concentration dependent and the free fraction increases from approximately 10% at 40 mcg/mL to 18.5% at 130 mcg/mL. Protein binding of valproate is reduced in the elderly, in patients with chronic hepatic diseases, in patients with renal impairment, and in the presence of other drugs (e.g., aspirin). Conversely, valproate may displace certain protein-bound drugs (e.g., phenytoin, carbamazepine, warfarin, and tolbutamide) [see DRUG INTERACTIONS( 7) for more detailed information on the pharmacokinetic interactions of valproate with other drugs].
CNS Distribution
Valproate concentrations in cerebrospinal fluid (CSF) approximate unbound concentrations in plasma (about 10% of total concentration).
Metabolism
Valproate is metabolized almost entirely by the liver. In adult patients on monotherapy, 30 to 50% of an administered dose appears in urine as a glucuronide conjugate. Mitochondrial β-oxidation is the other major metabolic pathway, typically accounting for over 40% of the dose. Usually, less than 15 to 20% of the dose is eliminated by other oxidative mechanisms. Less than 3% of an administered dose is excreted unchanged in urine.
The relationship between dose and total valproate concentration is nonlinear; concentration does not increase proportionally with the dose, but rather, increases to a lesser extent due to saturable plasma protein binding. The kinetics of unbound drug are linear.
Elimination
Mean plasma clearance and volume of distribution for total valproate are 0.56 L/hr/1.73 m 2and 11 L/1.73 m 2, respectively. Mean plasma clearance and volume of distribution for free valproate are 4.6 L/hr/1.73 m 2and 92 L/1.73 m 2. Mean terminal half-life for valproate monotherapy ranged from 9 to 16 hours following oral dosing regimens of 250 to 1,000 mg.
The estimates cited apply primarily to patients who are not taking drugs that affect hepatic metabolizing enzyme systems. For example, patients taking enzyme-inducing antiepileptic drugs (carbamazepine, phenytoin, and phenobarbital) will clear valproate more rapidly. Because of these changes in valproate clearance, monitoring of antiepileptic concentrations should be intensified whenever concomitant antiepileptics are introduced or withdrawn.
Special Populations
Effect of Age
Children
Pediatric patients (i.e., between 3 months and 10 years) have 50% higher clearances expressed on weight (i.e., mL/min/kg) than do adults. Over the age of 10 years, children have pharmacokinetic parameters that approximate those of adults.
Elderly
The capacity of elderly patients (age range: 68 to 89 years) to eliminate valproate has been shown to be reduced compared to younger adults (age range: 22 to 26 years). Intrinsic clearance is reduced by 39%; the free fraction is increased by 44%. Accordingly, the initial dosage should be reduced in the elderly [see DOSAGE AND ADMINISTRATION ( 2.2) ].
Effect of Sex
There are no differences in the body surface area adjusted unbound clearance between males and females (4.8±0.17 and 4.7±0.07 L/hr per 1.73 m 2, respectively).
Effect of Race
The effects of race on the kinetics of valproate have not been studied.
Effect of Disease
Liver Disease
Liver disease impairs the capacity to eliminate valproate. In one study, the clearance of free valproate was decreased by 50% in 7 patients with cirrhosis and by 16% in 4 patients with acute hepatitis, compared with 6 healthy subjects. In that study, the half-life of valproate was increased from 12 to 18 hours. Liver disease is also associated with decreased albumin concentrations and larger unbound fractions (2 to 2.6 fold increase) of valproate. Accordingly, monitoring of total concentrations may be misleading since free concentrations may be substantially elevated in patients with hepatic disease whereas total concentrations may appear to be normal [see BOXED WARNING, CONTRAINDICATIONS ( 4), WARNINGS AND PRECAUTIONS ( 5.1) ].
Renal Disease
A slight reduction (27%) in the unbound clearance of valproate has been reported in patients with renal failure (creatinine clearance < 10 mL/minute); however, hemodialysis typically reduces valproate concentrations by about 20%. Therefore, no dosage adjustment appears to be necessary in patients with renal failure. Protein binding in these patients is substantially reduced; thus, monitoring total concentrations may be misleading.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility - Carcinogenesis - Valproate was administered orally to rats and mice at doses of 80 and 170 mg/kg/day (less than the maximum ...Close
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
Valproate was administered orally to rats and mice at doses of 80 and 170 mg/kg/day (less than the maximum recommended human dose on a mg/m 2basis) for two years. The primary findings were an increase in the incidence of subcutaneous fibrosarcomas in high-dose male rats receiving valproate and a dose-related trend for benign pulmonary adenomas in male mice receiving valproate. The significance of these findings for humans is unknown.
Mutagenesis
Valproate was not mutagenic in an in vitrobacterial assay (Ames test), did not produce dominant lethal effects in mice, and did not increase chromosome aberration frequency in an in vivocytogenetic study in rats. Increased frequencies of sister chromatid exchange (SCE) have been reported in a study of epileptic children taking valproate, but this association was not observed in another study conducted in adults. There is some evidence that increased SCE frequencies may be associated with epilepsy. The biological significance of an increase in SCE frequency is not known.
Impairment of Fertility
Chronic toxicity studies of valproate in juvenile and adult rats and dogs demonstrated reduced spermatogenesis and testicular atrophy at oral doses of 400 mg/kg/day or greater in rats (approximately equivalent to or greater than the maximum recommended human dose (MRHD) on a mg/m 2basis) and 150 mg/kg/day or greater in dogs (approximately 1.4 times the MRHD or greater on a mg/m 2basis). Fertility studies in rats have shown no effect on fertility at oral doses of valproate up to 350 mg/kg/day (approximately equal to the MRHD on a mg/m 2basis) for 60 days. The effect of valproate on testicular development and on sperm parameters and fertility in humans is unknown.
-
14 CLINICAL STUDIES14.1 Epilepsy - The efficacy of valproate in reducing the incidence of complex partial seizures (CPS) that occur in isolation or in association with other seizure types was established in two ...Close
14.1 Epilepsy
The efficacy of valproate in reducing the incidence of complex partial seizures (CPS) that occur in isolation or in association with other seizure types was established in two controlled trials.
In one, multi-clinic, placebo-controlled study employing an add-on design, (adjunctive therapy) 144 patients who continued to suffer eight or more CPS per 8 weeks during an 8 week period of monotherapy with doses of either carbamazepine or phenytoin sufficient to assure plasma concentrations within the "therapeutic range" were randomized to receive, in addition to their original antiepilepsy drug (AED), either divalproex sodium or placebo. Randomized patients were to be followed for a total of 16 weeks. The following Table presents the findings.
Table 4 Adjunctive Therapy Study Median Incidence of CPS per 8 Weeks *Reduction from baseline statistically significantly greater for divalproex sodium than placebo at p ≤ 0.05 level.
Add on
TreatmentNumber of
PatientsBaseline
IncidenceExperimental
IncidenceDivalproex Sodium 75 16 8.9 * Placebo 69 14.5 11.5 Figure 1 presents the proportion of patients (X axis) whose percentage reduction from baseline in complex partial seizure rates was at least as great as that indicated on the Y axis in the adjunctive therapy study. A positive percent reduction indicates an improvement (i.e., a decrease in seizure frequency), while a negative percent reduction indicates worsening. Thus, in a display of this type, the curve for an effective treatment is shifted to the left of the curve for placebo. This Figure shows that the proportion of patients achieving any particular level of improvement was consistently higher for valproate than for placebo. For example, 45% of patients treated with valproate had a ≥ 50% reduction in complex partial seizure rate compared to 23% of patients treated with placebo.
Figure 1
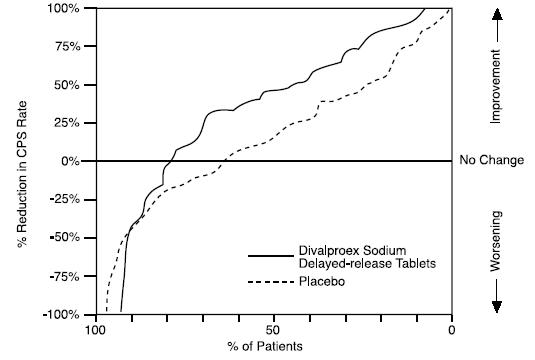
The second study assessed the capacity of valproate to reduce the incidence of CPS when administered as the sole AED. The study compared the incidence of CPS among patients randomized to either a high or low dose treatment arm. Patients qualified for entry into the randomized comparison phase of this study only if 1) they continued to experience 2 or more CPS per 4 weeks during an 8 to 12 week long period of monotherapy with adequate doses of an AED (i.e., phenytoin, carbamazepine, phenobarbital, or primidone) and 2) they made a successful transition over a two week interval to valproate. Patients entering the randomized phase were then brought to their assigned target dose, gradually tapered off their concomitant AED and followed for an interval as long as 22 weeks. Less than 50% of the patients randomized, however, completed the study. In patients converted to divalproex sodium delayed-release tablets monotherapy, the mean total valproate concentrations during monotherapy were 71 and 123 mcg/mL in the low dose and high dose groups, respectively.
The following Table presents the findings for all patients randomized who had at least one post-randomization assessment.
Table 5 Monotherapy Study Median Incidence of CPS per 8 Weeks *Reduction from baseline statistically significantly greater for high dose than low dose at p ≤ 0.05 level.
Treatment Number
of
PatientsBaseline
IncidenceRandomized
Phase
IncidenceHigh dose divalproex sodium delayed-release tablets 131 13.2 10.7 * Low dose divalproex sodium delayed-release tablets 134 14.2 13.8 Figure 2 presents the proportion of patients (X axis) whose percentage reduction from baseline in complex partial seizure rates was at least as great as that indicated on the Y axis in the monotherapy study. A positive percent reduction indicates an improvement (i.e., a decrease in seizure frequency), while a negative percent reduction indicates worsening. Thus, in a display of this type, the curve for a more effective treatment is shifted to the left of the curve for a less effective treatment. This Figure shows that the proportion of patients achieving any particular level of reduction was consistently higher for high dose valproate than for low dose valproate. For example, when switching from carbamazepine, phenytoin, phenobarbital or primidone monotherapy to high dose valproate monotherapy, 63% of patients experienced no change or a reduction in complex partial seizure rates compared to 54% of patients receiving low dose valproate.
Figure 2
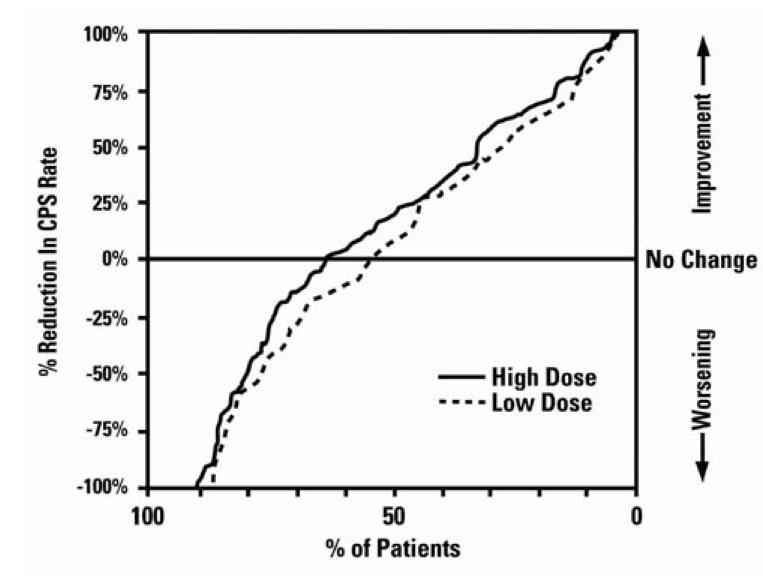
Information on pediatric studies is presented in section 8.
-
15 REFERENCES1. Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurology 2013; 12 ...
1. Meador KJ, Baker GA, Browning N, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurology 2013; 12 (3):244-252.
Close -
16 HOW SUPPLIED/STORAGE AND HANDLINGNDC 17856-0109-01 in a box of 100 Capsules - Divalproex sodium delayed-release capsules, USP (sprinkle) RX Only - 100 capsules - Storage: Store at 20° to 25°C (68° to 77°F) [See USP Controlled ...Close
NDC 17856-0109-01 in a box of 100 Capsules
Divalproex sodium delayed-release capsules, USP (sprinkle)
RX Only
100 capsules
Storage:
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Hepatotoxicity - Warn patients and guardians that nausea, vomiting, abdominal pain, anorexia, diarrhea ...Close
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Hepatotoxicity
Warn patients and guardians that nausea, vomiting, abdominal pain, anorexia, diarrhea, asthenia, and/or jaundice can be symptoms of hepatotoxicity and, therefore, require further medical evaluation promptly [see WARNINGS AND PRECAUTIONS ( 5.1) ].
Pancreatitis
Warn patients and guardians that abdominal pain, nausea, vomiting, and/or anorexia can be symptoms of pancreatitis and, therefore, require further medical evaluation promptly [see WARNINGS AND PRECAUTIONS( 5. 5) ].
Birth Defects and Decreased IQ
Inform pregnant women and women of childbearing potential that use of valproate during pregnancy increases the risk of birth defects and decreased IQ in children who were exposed. Advise women to use effective contraception while using valproate. When appropriate, counsel these patients about alternative therapeutic options. This is particularly important when valproate use is considered for a condition not usually associated with permanent injury or death. Advise patients to read the Medication Guide, which appears as the last section of the labeling [see WARNINGS AND PRECAUTIONS ( 5.2, 5.3, 5.4) AND USE IN SPECIFIC POPULATIONS ( 8.1)] .
Advise women of childbearing potential to discuss pregnancy planning with their doctor and to contact their doctor immediately if they think they are pregnant.
Encourage patients to enroll in the NAAED Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 [see USE IN SPECIFIC POPULATIONS ( 8.1) ].
Suicidal Thinking and Behavior
Counsel patients, their caregivers, and families that AEDs, including divalproex sodium, may increase the risk of suicidal thoughts and behavior and to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients, caregivers, and families to report behaviors of concern immediately to the healthcare providers [see WARNINGS AND PRECAUTIONS( 5.7) ].
Hyperammonemia
Inform patients of the signs and symptoms associated with hyperammonemic encephalopathy and to notify the prescriber if any of these symptoms occur [see WARNINGS AND PRECAUTIONS ( 5.9, 5.10) ].
CNS Depression
Since valproate products may produce CNS depression, especially when combined with another CNS depressant (e.g., alcohol), advise patients not to engage in hazardous activities, such as driving an automobile or operating dangerous machinery, until it is known that they do not become drowsy from the drug.
Multiorgan Hypersensitivity Reactions
Instruct patients that a fever associated with other organ system involvement (rash, lymphadenopathy, etc.) may be drug-related and should be reported to the physician immediately [see WARNINGS AND PRECAUTIONS ( 5.12) ].
Medication Residue in the Stool
Instruct patients to notify their healthcare provider if they notice a medication residue in the stool [seeWARNINGS AND PRECAUTIONS ( 5.18)].
Administration Guide
Divalproex sodium delayed-release capsules (sprinkle)
Divalproex sodium delayed-release capsules (sprinkle) may be swallowed whole or the capsule contents may be sprinkled onto soft food such as applesauce or pudding.
Serving suggestions
Divalproex sodium delayed-release capsules (sprinkle) provide the medicine that your healthcare provider has prescribed. The sprinkles are flavorless. Soft foods such as applesauce or pudding are best to use for mixing and taking divalproex sodium delayed-release capsules (sprinkle).
TO ADMINISTER WITH FOOD:

Make sure this medicine is taken exactly as your healthcare provider prescribed it. If you have any questions, please contact your healthcare provider or pharmacist. Keep all of your healthcare provider's appointments as scheduled. Make sure that divalproex sodium delayed-release capsules (sprinkle) and all other medicines are kept out of the reach of children.
Note:
You may see the specially coated particles of divalproex sodium delayed-release capsules (sprinkle) in stool. If you do, you should inform your healthcare provider.
Ask your healthcare provider or pharmacist about possible side effects with divalproex sodium delayed-release capsules (sprinkle).
Store divalproex sodium delayed-release capsules (sprinkle) at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
-
SPL UNCLASSIFIED SECTIONDistributed by: ATLANTIC BIOLOGICALS CORP. Miami, FL 33179
-
MEDICATION GUIDEMEDICATION GUIDE - Divalproex Sodium - (dye val PRO ex sew dee uhm) Delayed-release Capsules, USP(Sprinkle) Read this Medication Guide before you start taking divalproex sodium ...
Divalproex Sodium
(dye val PRO ex sew dee uhm)
Delayed-release Capsules, USP(Sprinkle)
Read this Medication Guide before you start taking divalproex sodium delayed-release capsules (sprinkle) and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know aboutdivalproex sodium delayed-release capsules (sprinkle)?
Do not stop takingdivalproex sodium delayed-release capsules (sprinkle)without first talking to your healthcare provider.
Stopping divalproex sodium delayed-release capsules (sprinkle) suddenly can cause serious problems.
Divalproex sodium delayed-release capsules (sprinkle)can cause serious side effects, including:
- Serious liver damage that can cause death, especially in children younger than 2 years old.
The risk of getting this serious liver damage is more likely to happen within the first 6 months of treatment.
Call your healthcare provider right away if you get any of the following symptoms:
- nausea or vomiting that does not go away
- loss of appetite
- pain on the right side of your stomach (abdomen)
- dark urine
- swelling of your face
- yellowing of your skin or the whites of your eyes
In some cases, liver damage may continue despite stopping the drug.
2 Divalproexsodium delayed-release capsules (sprinkle)may harm your unborn baby.
- If you take divalproex sodium delayed-release capsules (sprinkle) during pregnancy for any medical condition, your baby is at risk for serious birth defects that affect the brain and spinal cord and are called spina bifida or neural tube defects. These defects occur in 1 to 2 out of every 100 babies born to mothers who use this medicine during pregnancy. These defects can begin in the first month, even before you know you are pregnant. Other birth defects that affect the structures of the heart, head, arms, legs, and the opening where the urine comes out (urethra) on the bottom of the penis can also happen.
- Birth defects may occur even in children born to women who are not taking any medicines and do not have other risk factors.
- Taking folic acid supplements before getting pregnant and during early pregnancy can lower the chance of having a baby with a neural tube defect.
- If you take divalproex sodium delayed-release capsules (sprinkle) during pregnancy for any medical condition, your child is at risk for having a lower IQ.
- There may be other medicines to treat your condition that have a lower chance of causing birth defects and decreased IQ in your child.
- Women who are pregnant must not take divalproex sodium delayed-release capsules (sprinkle) to prevent migraine headaches.
- All women of childbearing age should talk to their healthcare provider about using other possible treatments instead ofdivalproex sodium delayed-release capsules (sprinkle). If the decision is made to use divalproex sodium delayed-release capsules (sprinkle), you should use effective birth control (contraception).
- Tell your healthcare provider right away if you become pregnant while taking divalproex sodium delayed-release capsules (sprinkle). You and your healthcare provider should decide if you will continue to take divalproex sodium delayed-release capsules (sprinkle) while you are pregnant.
- Pregnancy Registry: If you become pregnant while taking divalproex sodium delayed-release capsules (sprinkle), talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
3 Inflammation of your pancreas that can cause death.
Call your healthcare provider right away if you have any of these symptoms:
- severe stomach pain that you may also feel in your back
- nausea or vomiting that does not go away
4 Like other antiepileptic drugs, divalproex sodium delayed-release capsules (sprinkle) may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stopdivalproex sodium delayed-release capsules (sprinkle)without first talking to a healthcare provider.Stopping divalproex sodium delayed-release capsules (sprinkle) suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
What aredivalproex sodium delayed-release capsules (sprinkle)?
Divalproex sodium delayed-release capsules (sprinkle) are prescription medicines used alone or with other medicines, to treat:
- complex partial seizures in adults and children 10 years of age and older
- simple and complex absence seizures, with or without other seizure types
Who should not takedivalproex sodium delayed-release capsules (sprinkle)?
Do not take divalproex sodium delayed-release capsules (sprinkle) if you:
- have liver problems
- have or think you have a genetic liver problem caused by a mitochondrial disorder (e.g. Alpers-Huttenlocher syndrome)
- are allergic to divalproex sodium, valproic acid, sodium valproate, or any of the ingredients in divalproex sodium delayed-release capsules (sprinkle). See the end of this leaflet for a complete list of ingredients in divalproex sodium delayed-release capsules (sprinkle).
- have a genetic problem called urea cycle disorder
- are pregnant for the prevention of migraine headaches
What should I tell my healthcare provider before takingdivalproex sodium delayed-release capsules (sprinkle)?
Before you take divalproex sodium delayed-release capsules (sprinkle), tell your healthcare provider if you:
- have a genetic liver problem caused by a mitochondrial disorder (e.g. Alpers- Huttenlocher syndrome)
- drink alcohol
- are pregnant or breastfeeding. Divalproex sodium can pass into breast milk. Talk to your healthcare provider about the best way to feed your baby if you take divalproex sodium delayed-release capsules (sprinkle).
- have or have had depression, mood problems, or suicidal thoughts or behavior
- have any other medical conditions
Tell your healthcare provider about all the medicines you take,including prescription and non-prescription medicines, vitamins, herbal supplements and medicines that you take for a short period of time.
Taking divalproex sodium delayed-release capsules (sprinkle) with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist each time you get a new medicine.
How should I takedivalproex sodium delayed-release capsules (sprinkle)?
- Take divalproex sodium delayed-release capsules (sprinkle) exactly as your healthcare provider tells you. Your healthcare provider will tell you how much divalproex sodium delayed-release capsules (sprinkle) to take and when to take it.
- Your healthcare provider may change your dose.
- Do not change your dose of divalproex sodium delayed-release capsules (sprinkle) without talking to your healthcare provider.
- Do not stop takingdivalproex sodium delayed-release capsules (sprinkle)without first talking to your healthcare provider. Stopping divalproex sodium delayed-release capsules (sprinkle) suddenly can cause serious problems.
- Divalproex sodium delayed-release capsules (sprinkle) may be swallowed whole, or they may be opened and the contents may be sprinkled on a small amount of soft food, such as applesauce or pudding. See the Administration Guide at the end of this Medication Guide for detailed instructions on how to use divalproex sodium delayed-release capsules (sprinkle).
- If you take too much divalproex sodium delayed-release capsules (sprinkle), call your healthcare provider or local Poison Control Center right away.
What should I avoid while takingdivalproex sodium delayed-release capsules (sprinkle)?
- Divalproex sodium delayed-release capsules (sprinkle) can cause drowsiness and dizziness. Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking divalproex sodium delayed-release capsules (sprinkle), until you talk with your doctor. Taking divalproex sodium delayed-release capsules (sprinkle) with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive a car or operate dangerous machinery until you know how divalproex sodium delayed-release capsules (sprinkle) affects you. Divalproex sodium delayed-release capsules (sprinkle) can slow your thinking and motor skills.
What are the possible side effects ofdivalproex sodium delayed-release capsules (sprinkle)?
- See "What is the most important information I should know about divalproex sodium delayed-release capsules (sprinkle)?"
Divalproex sodium delayed-release capsules (sprinkle) can cause serious side effects including:
- Bleeding problems:red or purple spots on your skin, bruising, pain and swelling into your joints due to bleeding or bleeding from your mouth or nose.
- High ammonia levels in your blood:feeling tired, vomiting, changes in mental status.
- Low body temperature (hypothermia):drop in your body temperature to less than 950F, feeling tired, confusion, coma.
- Allergic (hypersensitivity) reactions:fever, skin rash, hives, sores in your mouth, blistering and peeling of your skin, swelling of your lymph nodes, swelling of your face, eyes, lips, tongue, or throat, trouble swallowing or breathing.
- Drowsiness or sleepiness in the elderly.This extreme drowsiness may cause you to eat or drink less than you normally would. Tell your doctor if you are not able to eat or drink as you normally do. Your doctor may start you at a lower dose of divalproex sodium delayed-release capsules (sprinkle).
Call your healthcare provider right away, if you have any of the symptoms listed above.
The common side effects ofdivalproex sodium delayed-release capsules (sprinkle)include:
- nausea
- headache
- sleepiness
- vomiting
- weakness
- tremor
- dizziness
- stomach pain
- blurry vision
- double vision
- diarrhea
- increased appetite
- weight gain
- hair loss
- loss of appetite
- problems with walking or coordination
These are not all of the possible side effects of divalproex sodium delayed-release capsules (sprinkle). For more information, ask your healthcare provider or pharmacist.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I storedivalproex sodium delayed-release capsules (sprinkle)?
- Store divalproex sodium delayed-release capsules (sprinkle) at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Keep divalproex sodium delayed-release capsules (sprinkle) and all medicines out of the reach of children.
General information about the safe and effective use ofdivalproex sodium delayed-release capsules (sprinkle)
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use divalproex sodium delayed-release capsules (sprinkle) for a condition for which it was not prescribed. Do not give divalproex sodium delayed-release capsules (sprinkle) to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about divalproex sodium delayed-release capsules (sprinkle). If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about divalproex sodium delayed-release capsules (sprinkle) that is written for health professionals.
Please address medical inquiries to, MedicalAffairs@zydususa.com or Tel.: 1-877-993-8779.
What are the ingredients indivalproex sodium delayed-release capsules (sprinkle)?
Active ingredient: divalproex sodium, USP
Inactive ingredients: colloidal silicon dioxide, FD &C blue # 1, gelatin, hypromellose, methacrylic acid copolymer dispersion, microcrystalline cellulose spheres, sodium lauryl sulfate, talc, titanium dioxide and triethyl citrate. Each capsule is printed with black pharmaceutical ink which contains: ammonia solution, butyl alcohol, dehydrated alcohol, ferrosoferric oxide, isopropyl alcohol, propylene glycol, potassium hydroxide, purified water and shellac.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
The product's labeling may have been updated. For latest package insert, please visit
Distributed by:
Atlantic Biologicals Corp.
Miami, FL 33179
Close -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

-
INGREDIENTS AND APPEARANCEProduct Information
DIVALPROEX SODIUM divalproex sodium capsule, coated pellets Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17856-0109(NDC:68382-106) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIVALPROEX SODIUM (UNII: 644VL95AO6) (VALPROIC ACID - UNII:614OI1Z5WI) VALPROIC ACID 125 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GELATIN (UNII: 2G86QN327L) HYPROMELLOSES (UNII: 3NXW29V3WO) ISOPROPYL ALCOHOL (UNII: ND2M416302) METHACRYLIC ACID (UNII: 1CS02G8656) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) WATER (UNII: 059QF0KO0R) Product Characteristics Color blue (BLUE) , white (WHITE) Score no score Shape CAPSULE (CAPSULE) Size 22mm Flavor Imprint Code ZA66;125mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17856-0109-1 100 in 1 BOX 01/28/2021 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078919 01/31/2019 Labeler - ATLANTIC BIOLOGICALS CORP. (047437707)
CloseEstablishment Name Address ID/FEI Business Operations ATLANTIC BIOLOGICALS CORP. 047437707 repack(17856-0109)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
DIVALPROEX SODIUM capsule, coated pellets
Number of versions: 3
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Apr 2, 2025 | 3 (current) | download |
| Jan 29, 2021 | 2 | download |
| Feb 1, 2019 | 1 | download |
RxNorm
DIVALPROEX SODIUM capsule, coated pellets
Under Review - Editing is pending for RxNorm. If in scope, these drugs will include RxNorm normal forms when editing is complete.
Get Label RSS Feed for this Drug
DIVALPROEX SODIUM capsule, coated pellets
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=699a9722-27bd-4e90-819b-d4ecb99466c8
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
DIVALPROEX SODIUM capsule, coated pellets
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 17856-0109-1 |

