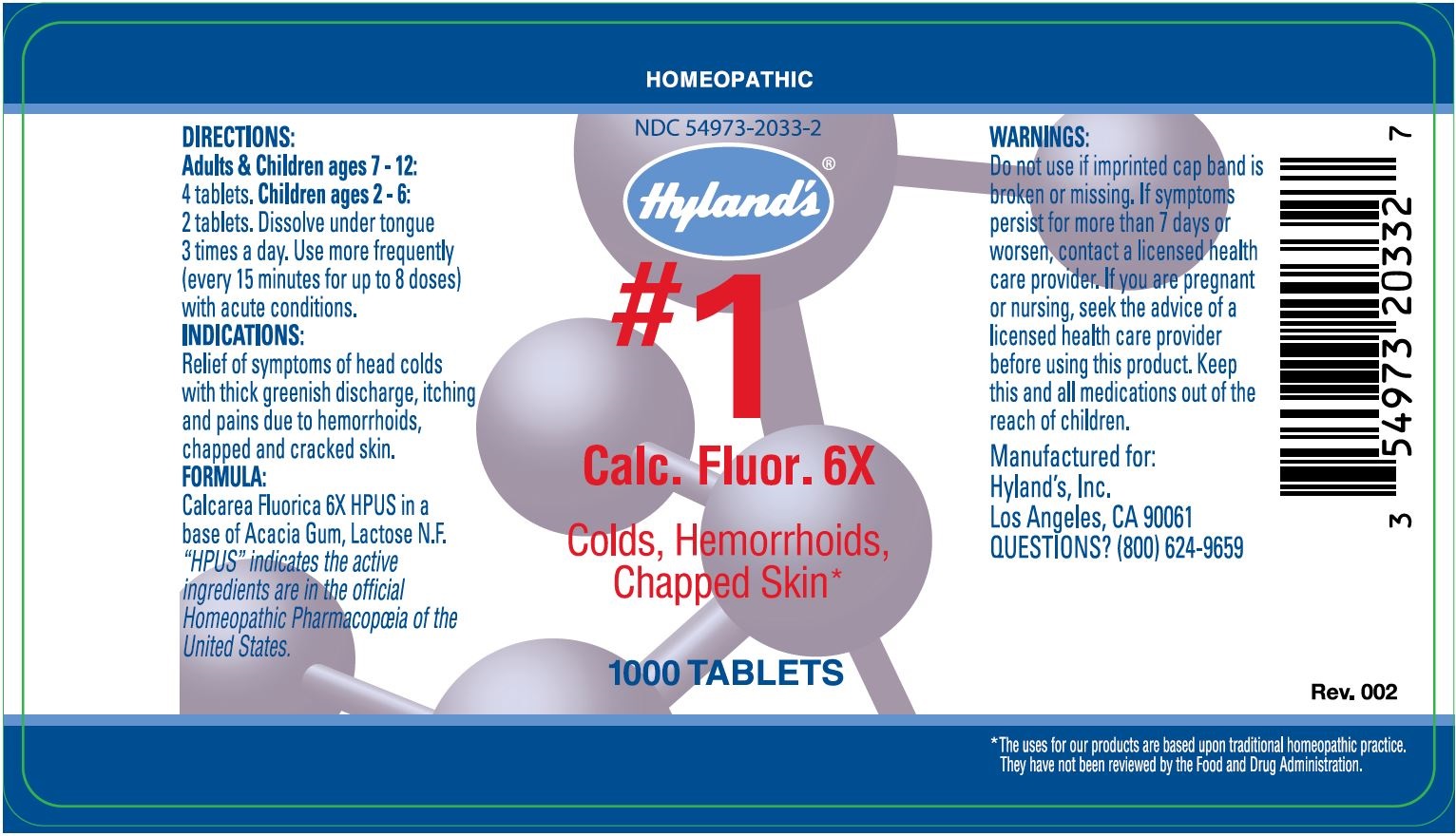
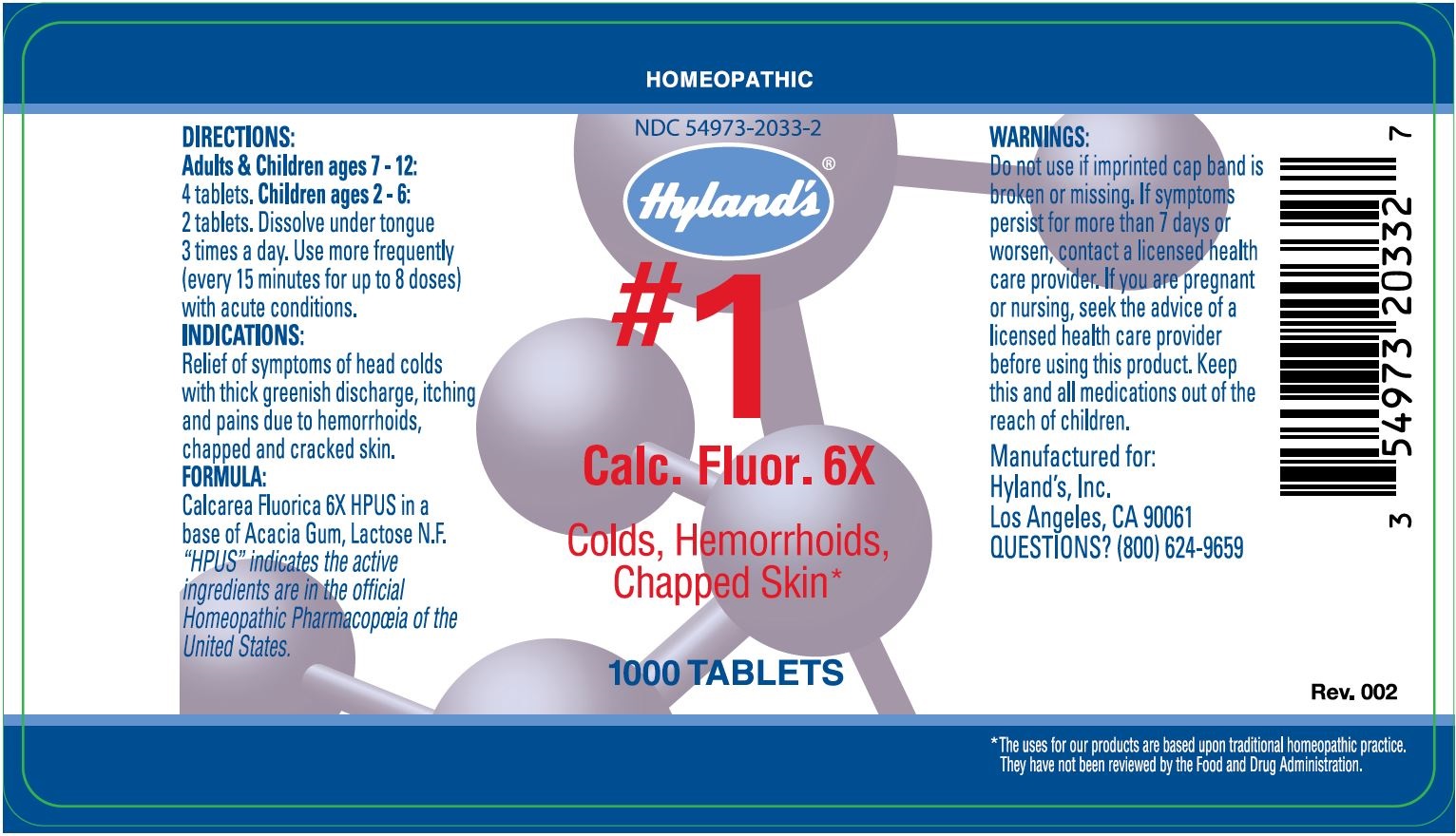
Label: CALC FLUOR- calcium fluoride tablet
- NDC Code(s): 54973-2033-1, 54973-2033-2
- Packager: Hyland's Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DIRECTIONSAdults & Children ages 6 - 12: 4 tablets. Children ages 2 - 6: 2 tablets. Dissolve under tongue 3 times a day. Use more frequently (every 15 minutes for up to 8 doses ...
-
PURPOSEFor Colds, Hemorrhoids, Chapped Skin - INDICATIONS - Relief of symptoms of headcolds with thick, greenish discharge. Relieves the symptoms of itching and burning pains due to hemorrhoids. Relieves ...
-
ACTIVE INGREDIENTCalcarea Fluorica 6X HPUS - In a base of Acacia Gum, Lactose N.F. “HPUS” indicates that the active ingredients are in the official Homeopathic Pharmacopœia of the United States.
-
WarningsDo not use if imprinted cap band is broken or missing. If symptoms persist for more than 7 days or worsen, contact a licensed health care provider. If you are pregnant or nursing, seek the ...
-
QUESTIONS?800.624.9659
-
PRINCIPAL DISPLAY PANEL - 1000 Tablet Bottle LabelHOMEOPATHIC - NDC 54973-2033-2 - Hyland's® #1 - Calc. Fluor. 6X - Colds, Hemorrhoids, Chapped Skin - 1000 TABLETS
-
INGREDIENTS AND APPEARANCEProduct Information