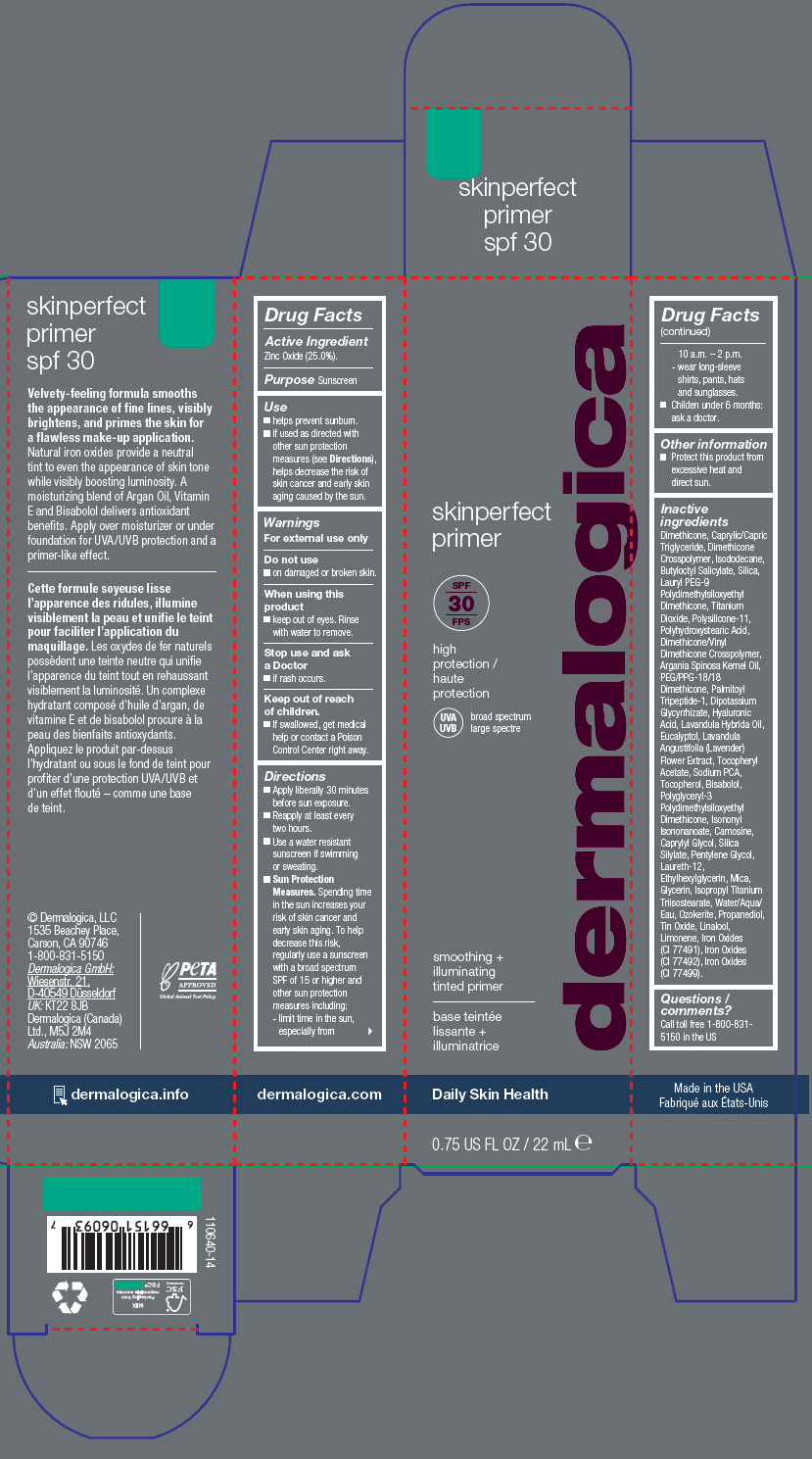
Label: SKINPERFECT PRIMER SPF30- zinc oxide lotion
- NDC Code(s): 68479-132-00, 68479-132-01, 68479-132-02
- Packager: Dermalogica, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
-
Use
- helps prevent sunburn.
- if used as directed with other sun protection measures (see Directions), helps decrease the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 30 minutes before sun exposure.
- Reapply at least every two hours.
- Use a water resistant sunscreen if swimming or sweating.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To help decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- -
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- -
- wear long-sleeve shirts, pants, hats and sunglasses.
- Childen under 6 months: ask a doctor.
- Other information
-
Inactive ingredients
Dimethicone, Caprylic/Capric Triglyceride, Dimethicone Crosspolymer, Isododecane, Butyloctyl Salicylate, Silica, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Titanium Dioxide, Polysilicone-11, Polyhydroxystearic Acid, Dimethicone/Vinyl Dimethicone Crosspolymer, Argania Spinosa Kernel Oil, PEG/PPG-18/18 Dimethicone, Palmitoyl Tripeptide-1, Dipotassium Glycyrrhizate, Hyaluronic Acid, Lavandula Hybrida Oil, Eucalyptol, Lavandula Angustifolia (Lavender) Flower Extract, Tocopheryl Acetate, Sodium PCA, Tocopherol, Bisabolol, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, Isononyl Isononanoate, Carnosine, Caprylyl Glycol, Silica Silylate, Pentylene Glycol, Laureth-12, Ethylhexylglycerin, Mica, Glycerin, Isopropyl Titanium Triisostearate, Water/Aqua/Eau, Ozokerite, Propanediol, Tin Oxide, Linalool, Limonene, Iron Oxides (CI 77491), Iron Oxides (CI 77492), Iron Oxides (CI 77499).
- Questions / comments?
- PRINCIPAL DISPLAY PANEL - 22 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
SKINPERFECT PRIMER SPF30
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68479-132 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 24.96 mg in 100 mL Inactive Ingredients Ingredient Name Strength Dimethicone (UNII: 92RU3N3Y1O) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Isododecane (UNII: A8289P68Y2) Butyloctyl Salicylate (UNII: 2EH13UN8D3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone (UNII: 25G622K2RA) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) Titanium Dioxide (UNII: 15FIX9V2JP) ARGAN OIL (UNII: 4V59G5UW9X) PEG/PPG-18/18 Dimethicone (UNII: 9H0AO7T794) Water (UNII: 059QF0KO0R) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S) (UNII: RLA2U05Z4Q) Mica (UNII: V8A1AW0880) Isononyl Isononanoate (UNII: S4V5BS6GCX) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LAVANDIN OIL (UNII: 9RES347CKG) LEVOMENOL (UNII: 24WE03BX2T) CERESIN (UNII: Q1LS2UJO3A) Pentylene Glycol (UNII: 50C1307PZG) Propanediol (UNII: 5965N8W85T) Carnosine (UNII: 8HO6PVN24W) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) Caprylyl Glycol (UNII: 00YIU5438U) Glycerin (UNII: PDC6A3C0OX) Isopropyl Titanium Triisostearate (UNII: 949E3KBJ1I) Ethylhexylglycerin (UNII: 147D247K3P) SODIUM PIDOLATE (UNII: 1V74VH163T) Laureth-12 (UNII: OAH19558U1) Hyaluronic Acid (UNII: S270N0TRQY) Eucalyptol (UNII: RV6J6604TK) STANNIC OXIDE (UNII: KM7N50LOS6) LAVANDULA ANGUSTIFOLIA SUBSP. ANGUSTIFOLIA FLOWER (UNII: 19AH1RAF4M) Tocopherol (UNII: R0ZB2556P8) Palmitoyl Tripeptide-1 (UNII: RV743D216M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68479-132-02 1 in 1 CARTON 12/02/2022 1 22 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:68479-132-01 1 in 1 CARTON 12/02/2022 2 7 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:68479-132-00 2 mL in 1 POUCH; Type 0: Not a Combination Product 12/02/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 12/02/2022 Labeler - Dermalogica, LLC. (177698560) Establishment Name Address ID/FEI Business Operations McKenna 090631412 MANUFACTURE(68479-132)