Label: GANCICLOVIR- ganciclovir sodium injection, powder, lyophilized, for solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 14335-030-01, 14335-030-25 - Packager: Hainan Poly Pharm. Co., Ltd.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 30, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use GANCICLOVIR for Injection, USP safely and effectively. See full prescribing information for GANCICLOVIR for Injection, USP ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HEMATOLOGIC TOXICITY, IMPAIRMENT OF FERTILITY, FETAL TOXICITY, MUTAGENESIS AND CARCINOGENESIS
- Hematologic Toxicity: Granulocytopenia, anemia, thrombocytopenia, and pancytopenia have been reported in patients treated with ganciclovir [see Warnings and Precautions ( 5.1)] .
- Impairment of Fertility: Based on animal data and limited human data, Ganciclovir for Injection, USP may cause temporary or permanent inhibition of spermatogenesis in males and suppression of fertility in females [see Warnings and Precautions ( 5.3)] . Impairment of Fertility: Based on animal data and limited human data, Ganciclovir for Injection, USP may cause temporary or permanent inhibition of spermatogenesis in males and suppression of fertility in females [see Warnings and Precautions ( 5.3)] .
- Fetal Toxicity: Based on animal data, Ganciclovir for Injection, USP has the potential to cause birth defects in humans [see Warnings and Precautions ( 5.4)] .
- Mutagenesis and Carcinogenesis: Based on animal data, Ganciclovir for Injection, USP has the potential to cause cancers in humans [see Warnings and Precautions ( 5.5)] .
-
1 INDICATIONS AND USAGE 1.1 Treatment of CMV Retinitis - Ganciclovir for Injection, USP is indicated for the treatment of cytomegalovirus (CMV) retinitis in immunocompromised adult patients, including patients with ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Important Dosing and Administration Information - To avoid phlebitis/pain at the infusion site, ganciclovir for injection must only be administered by intravenous infusion over 1 hour ...
-
3 DOSAGE FORMS AND STRENGTHS For injection: Single dose vial containing 500 mg of ganciclovir as a sterile lyophilized white to off-white powder for reconstitution with 10 mL of preservative-free Sterile Water for Injection ...
-
4 CONTRAINDICATIONS Ganciclovir for Injection, USP is contraindicated in patients who have experienced a clinically significant hypersensitivity reaction (e.g., anaphylaxis) to ganciclovir, valganciclovir or any ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Hematologic Toxicity - Granulocytopenia (neutropenia), anemia, thrombocytopenia and pancytopenia, have been observed in patients treated with ganciclovir. The frequency and severity of these ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed in greater detail in other sections of the labeling: Hematologic Toxicity - [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS Drug-drug interaction studies were conducted in patients with normal renal function. Patients with impaired renal function may have increased concentrations of ganciclovir and the coadministered ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - In animal studies, ganciclovir caused maternal and fetal toxicity and embryo-fetal mortality in pregnant mice and rabbits as well as teratogenicity in rabbits ...
-
10 OVERDOSAGE Reports of adverse reactions after overdoses with ganciclovir for injection, some with fatal outcomes, have been received from clinical trials and during post-marketing experience. One or more of ...
-
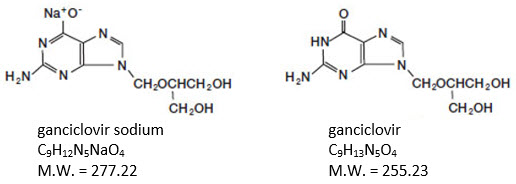
11 DESCRIPTION Ganciclovir for injection contains ganciclovir, in the form of the sodium salt for intravenous injection. Ganciclovir is a synthetic guanine derivative active against cytomegalovirus ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Ganciclovir is an antiviral drug with activity against CMV - [see Microbiology ( 12.4)] . 12.3 ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis, Mutagenesis - Ganciclovir was carcinogenic in mice at the same mean drug exposure in humans as at the RHD (5 mg/kg) ...
-
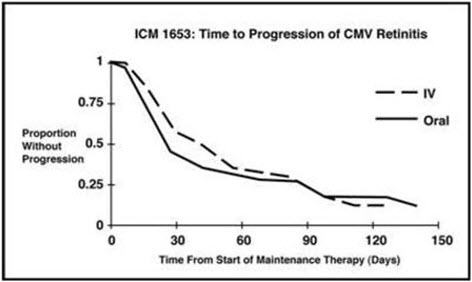
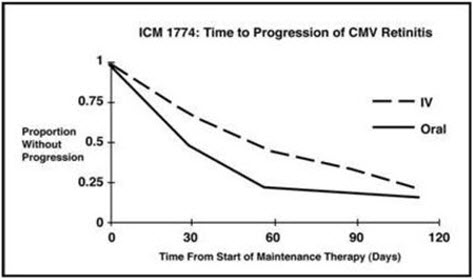
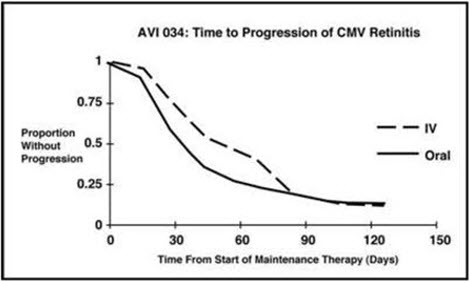
14 CLINICAL STUDIES 14.1 Treatment of CMV Retinitis - In a retrospective, non-randomized, single-center analysis of 41 patients with AIDS and CMV retinitis diagnosed by ophthalmologic examination between August ...
-
15 REFERENCES 1. “OSHA Hazardous Drugs.” OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
-
16 HOW SUPPLIED/STORAGE AND HANDLING How Supplied - Ganciclovir for Injection, USP is supplied in 10 mL sterile vials, each containing ganciclovir sodium equivalent to 500 mg of ganciclovir as a white to off-white powder. Ganciclovir ...
-
17 PATIENT COUNSELING INFORMATION Hematologic Toxicity - Inform patients that ganciclovir may cause hematologic toxicity including granulocytopenia (neutropenia), anemia, and thrombocytopenia. Inform patients that their blood ...
-
PRINCIPAL DISPLAY PANEL - VIAL LABEL NDC 14335-030-01 - Ganciclovir for - Injection, USP - 500 mg/vial - FOR INTRAVENOUS INFUSION ONLY - CAUTION: Handle this product - with great care because ...
-
INGREDIENTS AND APPEARANCEProduct Information