Label: NITROFURANTOIN suspension
- NDC Code(s): 70377-118-31
- Packager: Biocon Pharma Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NITROFURANTOIN ORAL SUSPENSION safely and effectively. See full prescribing information for NITROFURANTOIN ORAL ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGENitrofurantoin oral suspension is indicated in adults and pediatric patients 1 month of age and older for the treatment of urinary tract infections due to susceptible strains of Escherichia coli ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage and Administration in Adult Patients - The recommended dosage is 50 mg to 100 mg of nitrofurantoin oral suspension four times a day. For long-term suppressive therapy in ...
-
3 DOSAGE FORMS AND STRENGTHSNitrofurantoin oral suspension, USP is available as an opaque, yellow liquid oral suspension containing 25 mg/5 mL of nitrofurantoin.
-
4 CONTRAINDICATIONSNitrofurantoin oral suspension is contraindicated in: patients with known hypersensitivity to nitrofurantoin [see Warnings and Precautions (5.1)]. patients with a previous history of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving nitrofurantoin oral suspension [see Adverse ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in more detail in other sections of the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.1)] Pulmonary ...
-
7 DRUG INTERACTIONS7.1 Antacids - Antacids containing magnesium trisilicate, when administered concomitantly with nitrofurantoin, reduce both the rate and extent of absorption. The mechanism for this interaction ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Nitrofurantoin is contraindicated in pregnant women at term (38 weeks to 42 weeks gestation), during labor and delivery, or when the onset of labor is imminent ...
-
10 OVERDOSAGEIncidents of acute overdosage of nitrofurantoin oral suspension have resulted in symptoms such as vomiting. Induction of emesis is recommended. There is no specific antidote, but a high fluid ...
-
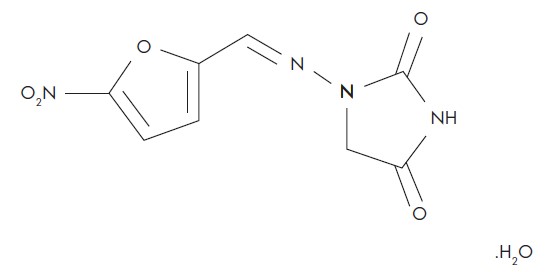
11 DESCRIPTIONNitrofurantoin Oral Suspension, USP contains nitrofurantoin, a synthetic nitrofuran antibacterial agent specific for urinary tract infections. The chemical name is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nitrofurantoin is an antibacterial drug [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Pharmacodynamic effects of nitrofurantoin oral suspension are ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Nitrofurantoin was not carcinogenic when fed to female Holtzman rats for 44.5 weeks or to female Sprague-Dawley rats ...
-
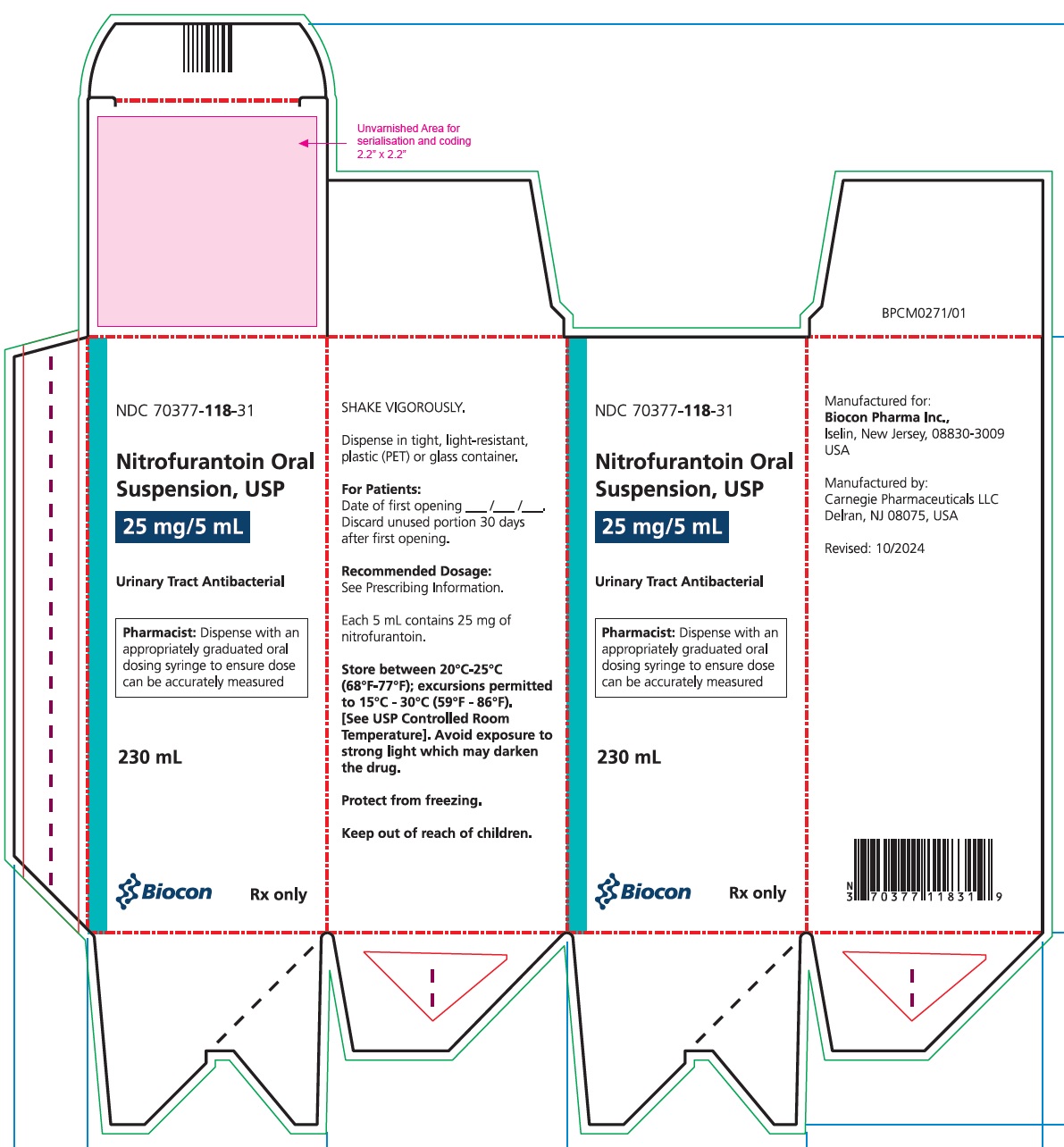
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Nitrofurantoin oral suspension, USP (25 mg/5 mL) is available in: NDC 70377-118-31 PET amber bottle of 230 mL - Storage and Handling - Avoid exposure to strong light which may darken ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions - Instruct the patients to: complete the full course of therapy; however, advise the patients or their caregiver to contact their physician if any unusual symptoms ...
-
PRINCIPAL DISPLAY PANELNDC 70377-118-31 - Nitrofurantoin Oral Suspension USP, 25 mg/5 mL - 230 mL - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information