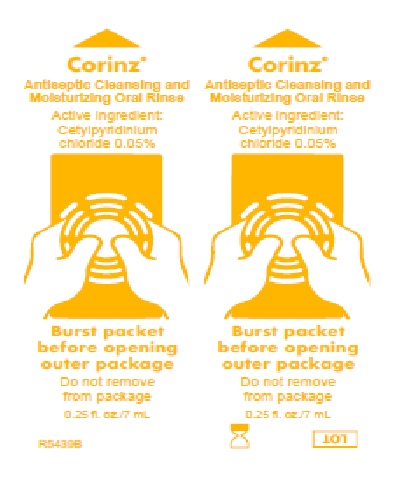
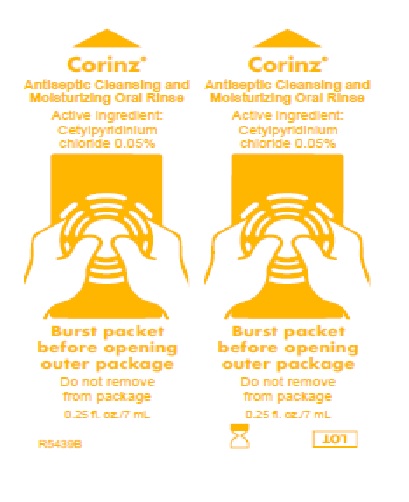
Label: CORINZ- cetylpyridinium chloride rinse
- NDC Code(s): 53462-375-07, 53462-375-30, 53462-375-60
- Packager: Sage Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Use
- INDICATIONS & USAGE
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- Directions
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Corinz
-
INGREDIENTS AND APPEARANCE
CORINZ
cetylpyridinium chloride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53462-375 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) XYLITOL (UNII: VCQ006KQ1E) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYSORBATE 20 (UNII: 7T1F30V5YH) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYDROXYETHYL CELLULOSE (4000 MPA.S AT 1%) (UNII: ZYD53NBL45) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SACCHARIN SODIUM (UNII: SB8ZUX40TY) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53462-375-60 7 mL in 1 PACKET; Type 0: Not a Combination Product 01/20/2015 2 NDC:53462-375-07 7 mL in 1 CUP; Type 0: Not a Combination Product 07/22/2019 3 NDC:53462-375-30 44 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/07/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 01/20/2015 Labeler - Sage Products, LLC (054326178) Registrant - Sage Products, LLC (054326178)