Label: DAYBUE- trofinetide solution
- NDC Code(s): 63090-660-01
- Packager: Acadia Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 2, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DAYBUE safely and effectively. See full prescribing information for DAYBUE. DAYBUE™ (trofinetide) oral solution - Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDAYBUE is indicated for the treatment of Rett syndrome in adults and pediatric patients 2 years of age and older.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - Administer DAYBUE orally twice daily, in the morning and evening, according to patient weight as shown in Table 1. DAYBUE can be taken with or without food. Table ...
-
3 DOSAGE FORMS AND STRENGTHSTrofinetide oral solution: 200 mg/mL of a pink to red, strawberry flavored solution.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Diarrhea - In Study 1 [see Clinical Studies (14)] and in long-term studies, 85% of patients treated with DAYBUE experienced diarrhea. In those treated with DAYBUE, 49% either had persistent ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: Diarrhea [see Warnings and Precautions (5.1)] Weight Loss [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Effect of DAYBUE on Other Drugs - Trofinetide is a weak CYP3A4 inhibitor; therefore, plasma concentrations of CYP3A4 substrates may be increased if given concomitantly with DAYBUE [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risks associated with the use of DAYBUE in pregnant women. No adverse developmental effects were observed ...
-
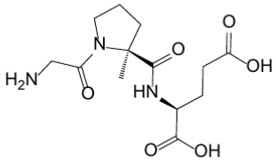
11 DESCRIPTIONTrofinetide is designated chemically as (2S)-2-{[(2S)-1-(2-aminoacetyl)-2-methylpyrrolidine-2-carbonyl]amino}pentanedioic acid (IUPAC). Its empirical formula is C13H21N3O6 and its molecular weight ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism by which trofinetide exerts therapeutic effects in patients with Rett syndrome is unknown. 12.2 Pharmacodynamics - Cardiac Electrophysiology - At ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Studies to evaluate the carcinogenic potential of trofinetide have not been ...
-
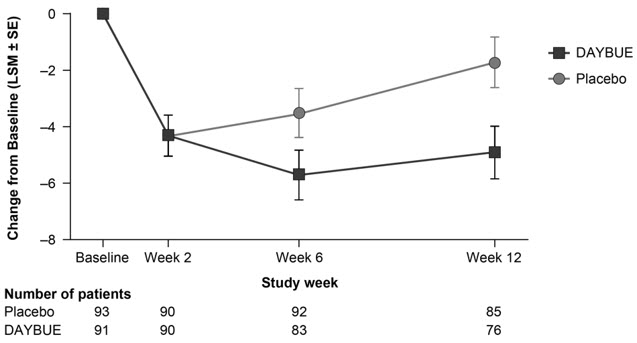
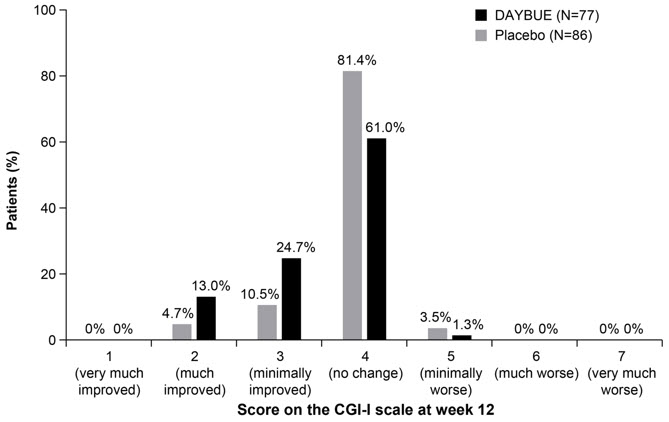
14 CLINICAL STUDIESThe efficacy of DAYBUE for the treatment of Rett syndrome was established in a 12-week randomized, double-blind, placebo-controlled study in patients with Rett syndrome 5 to 20 years of age (Study ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - DAYBUE (trofinetide) 200 mg/mL oral solution is a pink to red, strawberry flavored solution supplied in a nominal 500 mL round high-density polyethylene (HDPE) multi-dose ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the caregiver or patient to read the FDA-approved patient labeling (Patient Information). DAYBUE Administration - Advise the caregiver or patient that DAYBUE may be given orally or via ...
-
SPL UNCLASSIFIED SECTIONMarketed by: Acadia Pharmaceuticals Inc. San Diego, CA 92130 USA - DAYBUE is a trademark of Acadia Pharmaceuticals Inc. ©2024 Acadia Pharmaceuticals Inc. All rights reserved.
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - DAYBUE™ (day-BYOO) (trofinetide) oral solution - This Patient Information has been approved by the U.S. Food and Drug AdministrationApproved 9/2024 - What is ...
-
PRINCIPAL DISPLAY PANEL - 450 mL Bottle CartonNDC 63090-660-01 - Daybue™ (trofinetide) oral solution - 200 mg/mL - Recommended Dosage: See prescribing information. For oral or G-tube administration only. 450 mL - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information