Label: ATENOLOL tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 53002-4108-0, 53002-4108-3, 53002-4108-6 - Packager: RPK Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 64980-437
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 30, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
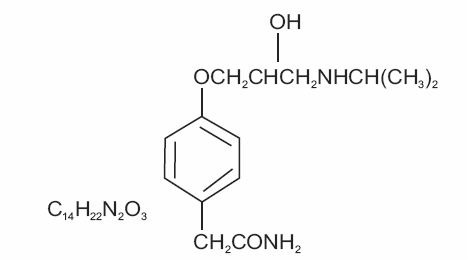
DESCRIPTIONAtenolol, a synthetic, beta1-selective (cardioselective) adrenoreceptor blocking agent, may be chemically described as benzeneacetamide, 4 -[2’-hydroxy-3’-[(1- methylethyl) amino] propoxy]-. The ...
-
CLINICAL PHARMACOLOGYAtenolol is a beta1-selective (cardioselective) beta-adrenergic receptor blocking agent without membrane stabilizing or intrinsic sympathomimetic (partial agonist) activities. This preferential ...
-
INDICATIONS & USAGEAtenolol tablets USP are indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events, primarily ...
-
CONTRAINDICATIONSAtenolol is contraindicated in sinus bradycardia, heart block greater than first degree, cardiogenic shock, and overt cardiac failure (see WARNINGS). Atenolol is contraindicated in those patients ...
-
WARNINGSCardiac Failure - Sympathetic stimulation is necessary in supporting circulatory function in congestive heart failure, and beta-blockade carries the potential hazard of further depressing ...
-
BOXED WARNING
(What is this?)
BOXED WARNING
Cessation of Therapy with Atenolol
Close
Patients with coronary artery disease, who are being treated with atenolol, should be advised against abrupt discontinuation of therapy. Severe exacerbation of angina and the occurrence of myocardial infarction and ventricular arrhythmias have been reported in angina patients following the abrupt discontinuation of therapy with beta blockers. The last two complications may occur with or without preceding exacerbation of the angina pectoris. As with other beta blockers, when discontinuation of atenolol is planned, the patients should be carefully observed and advised to limit physical activity to a minimum. If the angina worsens or acute coronary insufficiency develops, it is recommended that atenolol be promptly reinstituted, at least temporarily. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue atenolol therapy abruptly even in patients treated only for hypertension. (See Dosage and administration). -
SPL Unclassified SectionConcomitant Use of Calcium Channel Blockers - Bradycardia and heart block can occur and the left ventricular end diastolic pressure can rise when beta-blockers are administered with verapamil or ...
-
PRECAUTIONSGeneral - Patients already on a beta-blocker must be evaluated carefully before atenolol is administered. Initial and subsequent atenolol dosages can be adjusted downward depending on clinical ...
-
ADVERSE REACTIONSMost adverse effects have been mild and transient. The frequency estimates in the following table were derived from controlled studies in hypertensive patients in which adverse reactions were ...
-
OVERDOSAGEOverdosage with atenolol has been reported with patients surviving acute doses as high as 5 g. One death was reported in a man who may have taken as much as 10 g acutely. The predominant symptoms ...
-
DOSAGE & ADMINISTRATIONHypertension - The initial dose of atenolol is 50 mg given as one tablet a day either alone or added to diuretic therapy. The full effect of this dose will usually be seen within one to two weeks ...
-
HOW SUPPLIEDProduct: 53002-4108 - NDC: 53002-4108-3 30 TABLET in a BOTTLE - NDC: 53002-4108-6 60 TABLET in a BOTTLE - NDC: 53002-4108-0 100 TABLET in a BOTTLE
-
Atenolol 25mg Tablets

-
INGREDIENTS AND APPEARANCEProduct Information