Label: ANAGRELIDE HYDROCHLORIDE capsule
- NDC Code(s): 0172-5240-60, 0172-5241-60
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ANAGRELIDE CAPSULES safely and effectively. See full prescribing information for ANAGRELIDE CAPSULES. ANAGRELIDE capsules, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAnagrelide capsules are indicated for the treatment of patients with thrombocythemia, secondary to myeloproliferative neoplasms, to reduce the elevated platelet count and the risk of thrombosis ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Starting Dosage - Adults: The recommended starting dosage of anagrelide capsules is 0.5 mg four times daily or 1 mg twice daily. Pediatric Patients: The recommended starting ...
-
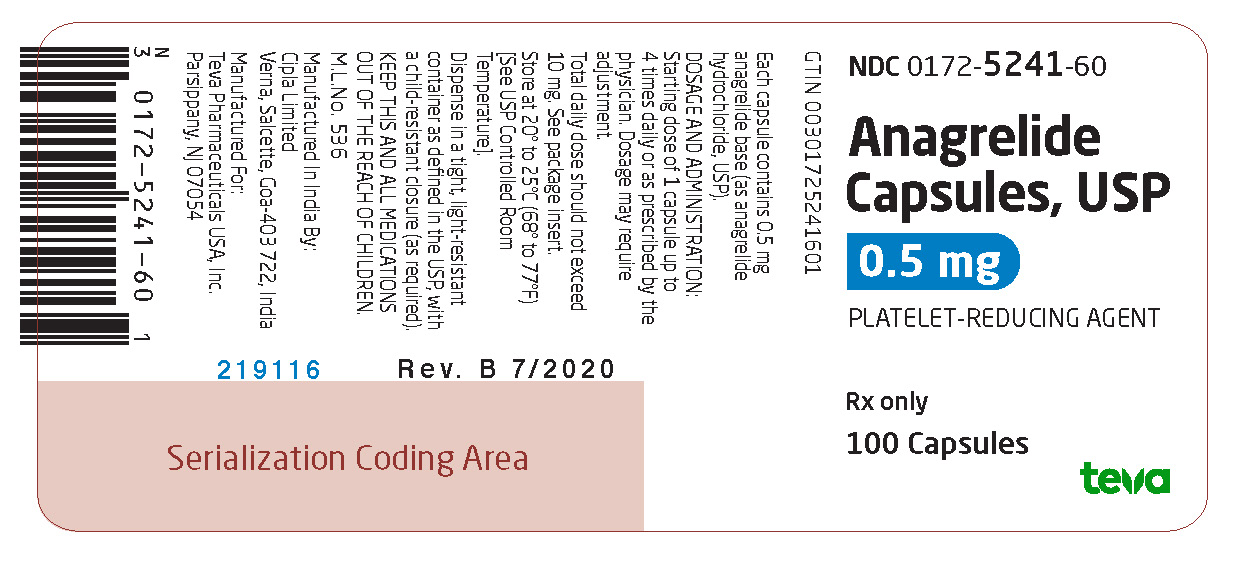
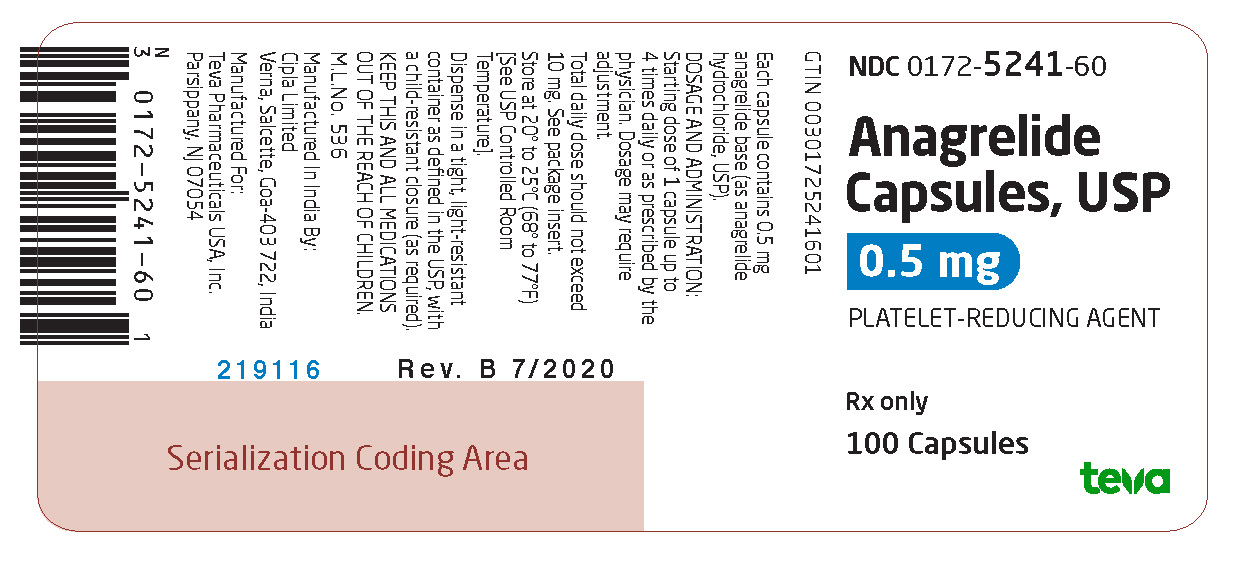
3 DOSAGE FORMS AND STRENGTHSAnagrelide Capsules USP, 0.5 mg are available as light gray opaque cap/white opaque body hard gelatin capsules, spin printed in black ink “Ivax hourglass logo” “5241” on the cap and “0.5 mg” on ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular Toxicity - Torsades de pointes and ventricular tachycardia have been reported with anagrelide. Obtain a pre-treatment cardiovascular examination including an ECG in all ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling: Cardiovascular Toxicity [see Warnings and Precautions (5.1)] Pulmonary ...
-
7 DRUG INTERACTIONS7.1 Drugs that Prolong QT - Avoid use of anagrelide in patients taking medications that may prolong QT interval (including, but not limited to, chloroquine, clarithromycin, haloperidol ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from case reports with anagrelide use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse ...
-
10 OVERDOSAGEAt higher than recommended doses, anagrelide has been shown to cause hypotension. There have been postmarketing case reports of intentional overdose with anagrelide hydrochloride. Reported ...
-
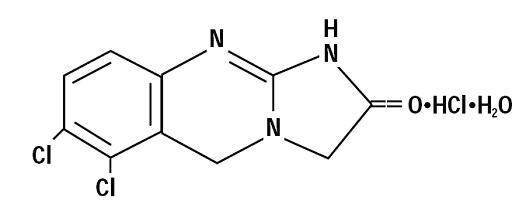
11 DESCRIPTIONAnagrelide hydrochloride, USP is a platelet-reducing agent. Its chemical name is 6,7-dichloro-1,5-dihydroimidazo[2,1-b] quinazolin-2(3H)-one monohydrochloride monohydrate and it has the following ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism by which anagrelide reduces blood platelet count is unknown. In cell culture studies, anagrelide suppressed expression of transcription factors ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a two-year rat carcinogenicity study a higher incidence of uterine adenocarcinoma, relative to controls, was observed in females ...
-
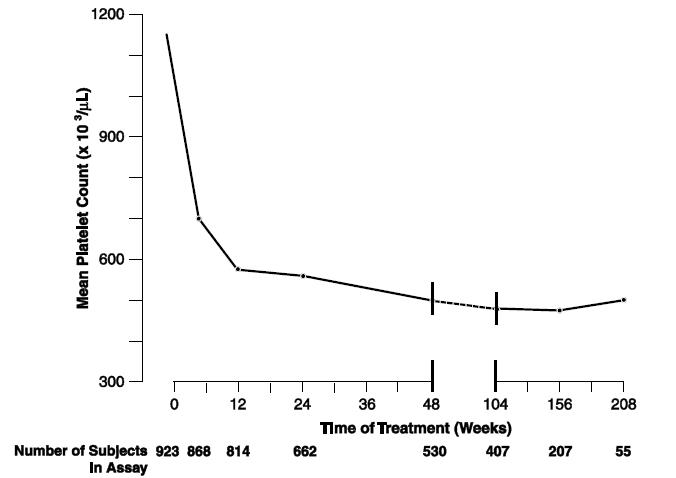
14 CLINICAL STUDIESClinical Studies in Adult Patients: A total of 942 patients with myeloproliferative neoplasms including 551 patients with Essential Thrombocythemia (ET), 117 patients with Polycythemia Vera ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAnagrelide Capsules USP, 0.5 mg are available as light gray opaque cap/white opaque body hard gelatin capsules, spin printed in black ink “Ivax hourglass logo” “5241” on the cap and “0.5 mg” on ...
-
17 PATIENT COUNSELING INFORMATIONDose: Tell the patient that their dose will be adjusted on a weekly basis until they are on a dose that lowers their platelets to an appropriate level. This will also help the patient to adjust ...
-
Package/Label Display PanelNDC 0172-5241-60 - Anagrelide Capsules, USP - 0.5 mg - PLATELET-REDUCING AGENT - Rx only - 100 Capsules
-
Package/Label Display PanelNDC 0172-5240-60 - Anagrelide Capsules, USP - 1 mg - PLATELET-REDUCING AGENT - Rx only - 100 Capsules
-
INGREDIENTS AND APPEARANCEProduct Information