Label: IWILFIN- eflornithine hydrochloride tablet
- NDC Code(s): 78670-150-01
- Packager: USWM, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IWILFIN® safely and effectively. See full prescribing information for IWILFIN. IWILFIN® (eflornithine) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEIWILFIN (eflornithine) is indicated to reduce the risk of relapse in adult and pediatric patients with high-risk neuroblastoma (HRNB) who have demonstrated at least a partial response to prior ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Testing Before Initiating IWILFIN - Prior to initiating IWILFIN, perform complete blood count, liver function tests, and baseline audiogram [see Warnings and Precautions ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 192 mg eflornithine, white to off-white, round, imprinted with "EFL" on one side and "192" on the other side.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - IWILFIN can cause myelosuppression. In the pooled safety population [see Adverse Reactions (6.1)], Grade 3 or 4 neutropenia occurred in 4.2% of patients. Febrile ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Myelosuppression [see Warnings and Precautions (5.1)] Hepatotoxicity [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1)], IWILFIN can cause fetal harm when administered to a ...
-
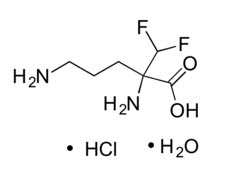
11 DESCRIPTIONIWILFIN is an ornithine decarboxylase inhibitor. The chemical name of eflornithine hydrochloride is 2,5-diamino-2-(difluoromethyl) pentanoic acid hydrochloride hydrate with a molecular formula of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Eflornithine is an irreversible inhibitor of the enzyme ornithine decarboxylase (ODC), the first and rate-limiting enzyme in the biosynthesis of polyamines and a ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a 2-year carcinogenicity study, once daily oral administration of eflornithine to female rats did not result in drug-related ...
-
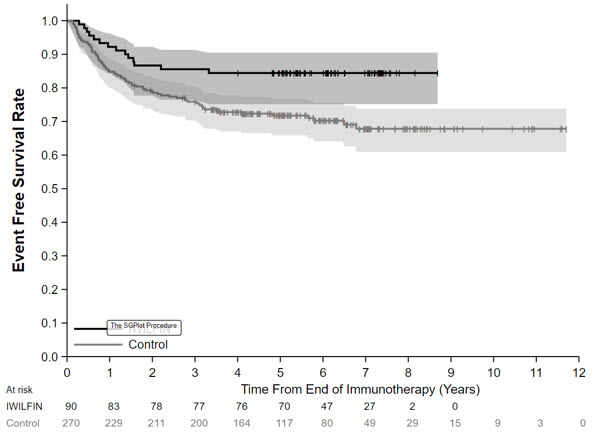
14 CLINICAL STUDIESThe efficacy of IWILFIN is based on an externally controlled trial comparison of Study 3b (investigational arm) and Study ANBL0032 (clinical trial-derived external control arm). Study ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGIWILFIN (eflornithine) is available as 192 mg round, white to off-white tablets imprinted with EFL on one side and 192 on the other side; approximately 11 mm in diameter and supplied as ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Myelosuppression - Inform patients and caregivers of the risk of bone marrow suppression and to promptly ...
-
SPL UNCLASSIFIED SECTIONUS WorldMeds® Distributed by: USWM, LLC - 4441 Springdale Road - Louisville, KY 40241 - ©2025. IWILFIN® is a registered trademark of USWM, LLC. FPI-0026.1
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug AdministrationIssued: 12/2023 - Patient Information - IWILFIN® (I-WILL-fin) (eflornithine) tablets - What is ...
-
PRINCIPAL DISPLAY PANEL - 192 mg Bottle CartonRx only - NDC 78670-150-01 - iwilfin™ (eflornithine) tablets - 192 mg - Keep the bottle tightly closed. 100 tablets - US WorldMeds®
-
INGREDIENTS AND APPEARANCEProduct Information