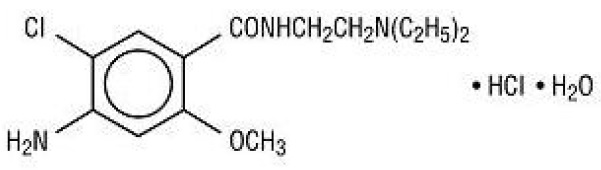Label: METOCLOPRAMIDE tablet
- NDC Code(s): 70518-0669-0
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 0093-2203
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METOCLOPRAMIDE TABLETS safely and effectively. See full prescribing information for METOCLOPRAMIDE TABLETS. METOCLOPRAMIDE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: TARDIVE DYSKINESIA
- Metoclopramide can cause tardive dyskinesia (TD), a serious movement disorder that is often irreversible. There is no known treatment for TD. The risk of developing TD increases with duration of treatment and total cumulative dosage [ see Warnings and Precautions ( 5.1) ].
- Discontinue metoclopramide in patients who develop signs or symptoms of TD. In some patients, symptoms may lessen or resolve after metoclopramide is stopped [ see Warnings and Precautions ( 5.1) ].
- Avoid treatment with metoclopramide for longer than 12 weeks because of the increased risk of developing TD with longer-term use [ see Warnings and Precautions (5.1) and Dosage and Administration ( 2.2, 2.3) ].
-
1 INDICATIONS AND USAGEMetoclopramide tablets are indicated for the: Treatment for 4 to 12 weeks of symptomatic, documented gastroesophageal reflux in adults who fail to respond to conventional therapy. Relief of ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Avoid treatment with metoclopramide for longer than 12 weeks because of the increased risk of developing TD with longer-term use [ see ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 10 mg metoclopramide, USP: white, round, scored, debossed “TEVA” on one side and “2203” above the score on the other side.
-
4 CONTRAINDICATIONSMetoclopramide is contraindicated: In patients with a history of tardive dyskinesia (TD) or a dystonic reaction to metoclopramide [ see Warnings and Precautions (5.1 ...
-
5 WARNINGS AND PRECAUTIONS5.1 Tardive Dyskinesia - Metoclopramide can cause tardive dyskinesia (TD), a syndrome of potentially irreversible and disfiguring involuntary movements of the face or tongue, and sometimes of the ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described, or described in greater detail, in other sections of the labeling: Tardive dyskinesia [ see - Boxed Warning and ...
-
7 DRUG INTERACTIONS7.1 Effects of Other Drugs on Metoclopramide - Table 3 displays the effects of other drugs on metoclopramide. Table 3. Effects of Other Drugs on ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Published studies, including retrospective cohort studies, national registry studies, and meta-analyses, do not report an increased risk of adverse ...
-
10 OVERDOSAGEManifestations of metoclopramide overdosage included drowsiness, disorientation, extrapyramidal reactions, other adverse reactions associated with metoclopramide use (including, e.g. ...
-
11 DESCRIPTIONMetoclopramide hydrochloride, USP, the active ingredient of metoclopramide tablets, USP is a dopamine-2 receptor antagonist. Metoclopramide hydrochloride (metoclopramide monohydrochloride ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Metoclopramide stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. The exact mechanism of action of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - A 77-week study was conducted in rats with oral metoclopramide doses up to 40 mg/kg/day (about six times the maximum ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach white, round, scored, debossed “TEVA” on one side and “2203” above the score on the other side, compressed metoclopramide tablet, USP contains metoclopramide hydrochloride, USP equivalent to ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Inform patients or their caregivers that metoclopramide tablets can cause serious adverse reactions. Instruct ...
-
MEDICATION GUIDEMetoclopramide (met''oh kloe'pra mide) Tablets - Read this Medication Guide before you start taking metoclopramide tablets and each time you get a refill. There may be new information ...
-
PRINCIPAL DISPLAY PANELDRUG: Metoclopramide - GENERIC: Metoclopramide - DOSAGE: TABLET - ADMINSTRATION: ORAL - NDC: 70518-0669-00 - COLOR: white - SHAPE: ROUND - SCORE: Two even pieces - SIZE: 8 mm - IMPRINT: TEVA;2203 - PACKAGING: 30 in 1 ...
-
INGREDIENTS AND APPEARANCEProduct Information