Label: SOFTSOAP ANTIBACTERIAL WITH MOISTURIZERS CRISP CLEAN- benzalkonium chloride liquid
-
NDC Code(s):
35000-086-11,
35000-086-20,
35000-086-26,
35000-086-31, view more35000-086-34, 35000-086-55, 35000-086-73, 35000-086-75, 35000-086-80, 35000-086-91
- Packager: Colgate-Palmolive Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1.47 L Bottle Label
-
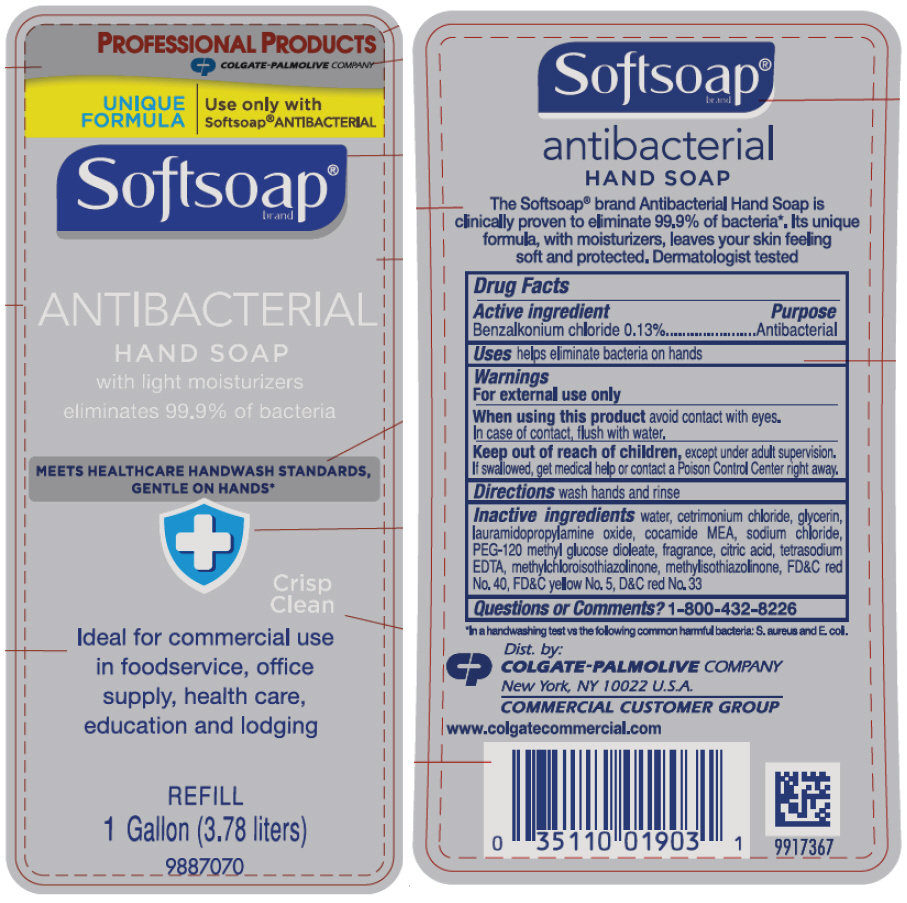
PRINCIPAL DISPLAY PANEL - 3.78 liter Bottle Label
PROFESSIONAL PRODUCTS
COLGATE-PALMOLIVE COMPANY
UNIQUE
FORMULAUse only with
Softsoap® ANTIBACTERIALSoftsoap®
brandANTIBACTERIAL
HAND SOAP
with light moisturizers
eliminates 99.9% of bacteriaMEETS HEALTHCARE HANDWASH STANDARDS,
GENTLE ON HANDS*Crisp
CleanIdeal for commercial use
in foodservice, office
supply, health care,
education and lodgingREFILL
1 Gallon (3.78 liters)9887070
- PRINCIPAL DISPLAY PANEL - 591 ml Bottle Label
-
INGREDIENTS AND APPEARANCE
SOFTSOAP ANTIBACTERIAL WITH MOISTURIZERS CRISP CLEAN
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:35000-086 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.33 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) GLYCERIN (UNII: PDC6A3C0OX) LAURAMIDOPROPYLAMINE OXIDE (UNII: I6KX160QTV) COCO MONOETHANOLAMIDE (UNII: C80684146D) SODIUM CHLORIDE (UNII: 451W47IQ8X) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE SODIUM (UNII: MP1J8420LU) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Product Characteristics Color ORANGE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35000-086-91 1478 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/04/2019 2 NDC:35000-086-75 221 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2013 3 NDC:35000-086-26 162 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2013 4 NDC:35000-086-11 332 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2013 5 NDC:35000-086-31 946 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2013 6 NDC:35000-086-55 1650 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2013 7 NDC:35000-086-34 3780 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2013 8 NDC:35000-086-80 1 in 1 CARTON 01/19/2013 12/31/2018 8 800 mL in 1 BAG; Type 0: Not a Combination Product 9 NDC:35000-086-73 1890 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/19/2013 10 NDC:35000-086-20 591 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/11/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG 505G(a)(3) 01/19/2013 Labeler - Colgate-Palmolive Company (001344381) Establishment Name Address ID/FEI Business Operations Colgate-Palmolive Company 079342773 ANALYSIS(35000-086) , MANUFACTURE(35000-086) , PACK(35000-086) , LABEL(35000-086) Establishment Name Address ID/FEI Business Operations Veepak, Inc. 874763303 MANUFACTURE(35000-086)





