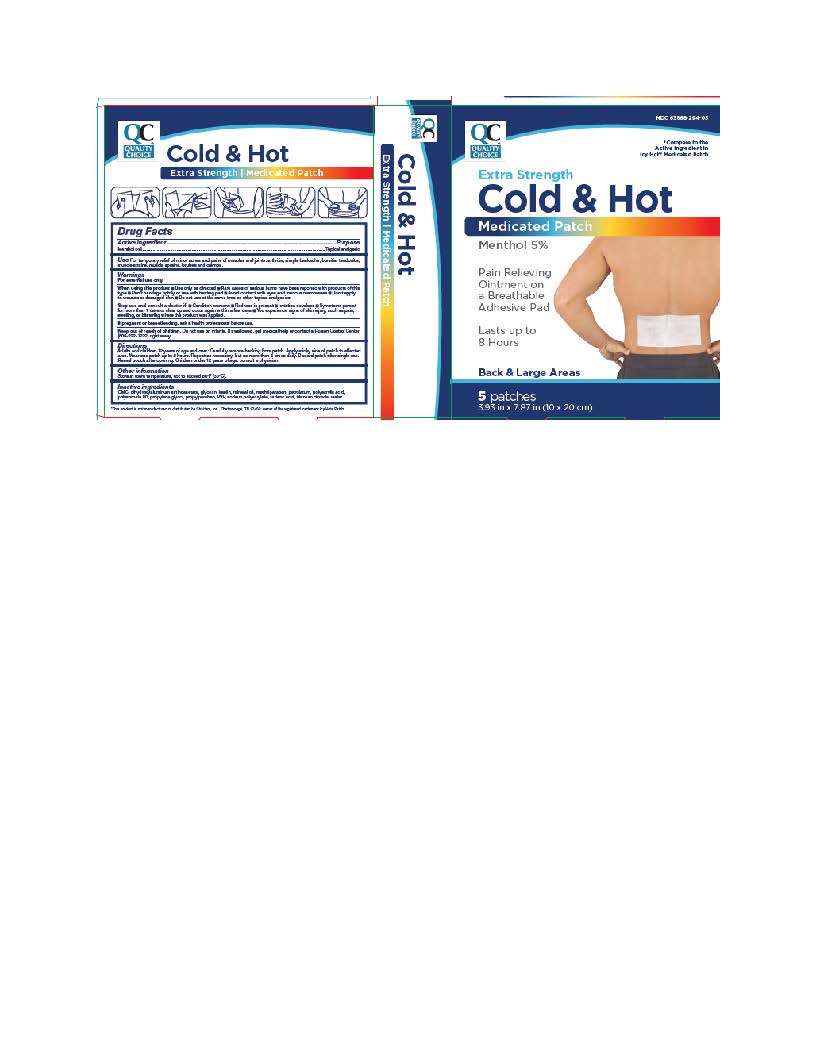
Label: BACK AND LARGE EXTRA STRENGTH COLD-HOT MEDICATED PATCHES- menthol patch
- NDC Code(s): 63868-294-05
- Packager: QUALITY CHOICE (Chain Drug Marketing Association)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Adults and children 12 years of age and over: Carefully remove backing from patch. Apply sticky side of patch to affected area. Wear one patch up to 8 hours. Repeat as necessary, but no more than 4 times daily. Reseal pouch after opening. Discard patch after single use. Children under 12 years of age: consult a physician.
- PURPOSE
- When using this product
- Stop use and ask a doctor
- If pregnant or breastfeeding
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BACK AND LARGE EXTRA STRENGTH COLD-HOT MEDICATED PATCHES
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-294 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) GLYCERIN (UNII: PDC6A3C0OX) KAOLIN (UNII: 24H4NWX5CO) METHYLPARABEN (UNII: A2I8C7HI9T) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) TARTARIC ACID (UNII: W4888I119H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-294-05 5 in 1 BOX 01/01/2019 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2019 Labeler - QUALITY CHOICE (Chain Drug Marketing Association) (011920774) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co., Ltd., 529128763 manufacture(63868-294) Establishment Name Address ID/FEI Business Operations Beijing HKKY Medical 544434817 manufacture(63868-294)