Label: TEMAZEPAM capsule
- NDC Code(s): 70518-0647-0, 70518-0647-1, 70518-0647-2
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 67877-146
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation.
The use of benzodiazepines, including temazepam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing temazepam and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see WARNINGS).
The continued use of benzodiazepines, including Temazepam, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of temazepam after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue temazepam or reduce the dosage (see DOSAGE AND ADMINISTRATIONand WARNINGS).
Close -
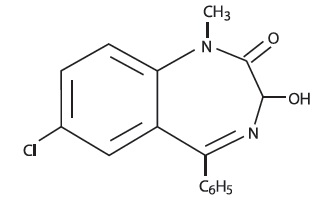
DESCRIPTIONTemazepam is a benzodiazepine hypnotic agent. The chemical name is 7-chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-2H-1, 4-benzodiazepin-2-one, and the structural formula is: C - 16H ...
-
CLINICAL PHARMACOLOGYPharmacokinetics - In a single and multiple dose absorption, distribution, metabolism, and excretion (ADME) study, using - 3H labeled drug, temazepam was well absorbed and found to have minimal ...
-
INDICATIONS & USAGETemazepam Capsules, USP are indicated for the short-term treatment of insomnia (generally 7 to 10 days). For patients with short-term insomnia, instructions in the prescription should indicate ...
-
WARNINGSRisks from Concomitant Use with Opioids - Concomitant use of benzodiazepines, including Temazepam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of ...
-
PRECAUTIONSGeneral - Since the risk of the development of oversedation, dizziness, confusion, and/or ataxia increases substantially with larger doses of benzodiazepines in elderly and debilitated patients ...
-
ADVERSE REACTIONSDuring controlled clinical studies in which 1076 patients received temazepam at bedtime, the drug was well tolerated. Side effects were usually mild and transient. Adverse reactions occurring in ...
-
DRUG ABUSE AND DEPENDENCEControlled Substance - Temazepam Capsules contains temazepam, a Schedule IV controlled substance. Abuse - Temazepam is a benzodiazepine and a CNS depressant with a potential for abuse and ...
-
OVERDOSAGEOverdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion ...
-
DOSAGE & ADMINISTRATIONWhile the recommended usual adult dose is 15 mg before retiring, 7.5 mg may be sufficient for some patients, and others may need 30 mg. In transient insomnia, a 7.5 mg dose may be sufficient to ...
-
HOW SUPPLIEDTemazepam Capsules USP - 15 mg - Blue opaque cap and white opaque body, imprinted “15 mg” on cap and “Novel 121” on the body in black ink. NDC: 70518-0647-00 - NDC: 70518-0647-01 - NDC ...
-
MEDICATION GUIDETEMAZEPAM (tem az' e pam) Capsules, C-IV - What is the most important information I should know about temazepam? temazepam is a benzodiazepine medicine. Taking benzodiazepines with opioid ...
-
PRINCIPAL DISPLAY PANELDRUG: temazepam - GENERIC: temazepam - DOSAGE: CAPSULE - ADMINSTRATION: ORAL - NDC: 70518-0647-0 - NDC: 70518-0647-1 - NDC: 70518-0647-2 - COLOR: blue - SHAPE: CAPSULE - SCORE: No score - SIZE: 19 mm - IMPRINT ...
-
INGREDIENTS AND APPEARANCEProduct Information