Label: DACARBAZINE injection, powder, for solution
- NDC Code(s): 71288-174-20, 71288-174-21
- Packager: Meitheal Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
It is recommended that dacarbazine for injection be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents.
- Hemopoietic depression is the most common toxicity with dacarbazine for injection. (See WARNINGS.)
- Hepatic necrosis has been reported. (See WARNINGS.)
- Studies have demonstrated this agent to have a carcinogenic and teratogenic effect when used in animals.
- In treatment of each patient, the physician must weigh carefully the possibility of achieving therapeutic benefit against the risk of toxicity.
-
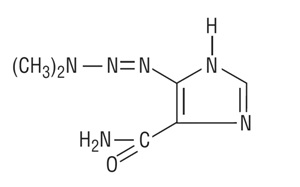
DESCRIPTIONDacarbazine for Injection, USP is a white to an ivory colored solid which is light sensitive. Each 20 mL vial contains 200 mg of dacarbazine, USP (active ingredient). Each vial also contains ...
-
CLINICAL PHARMACOLOGYAfter intravenous administration of dacarbazine for injection, the volume of distribution exceeds total body water content suggesting localization in some body tissue, probably the liver. Its ...
-
INDICATIONS AND USAGEDacarbazine for Injection is indicated in the treatment of metastatic malignant melanoma. In addition, Dacarbazine for Injection is also indicated for Hodgkin's disease as a secondary-line therapy ...
-
CONTRAINDICATIONSDacarbazine for injection is contraindicated in patients who have demonstrated a hypersensitivity to it in the past.
-
WARNINGSHemopoietic depression is the most common toxicity with dacarbazine for injection and involves primarily the leukocytes and platelets, although anemia may sometimes occur. Leukopenia and ...
-
PRECAUTIONSHospitalization is not always necessary but adequate laboratory study capability must be available. Extravasation of the drug subcutaneously during intravenous administration may result in tissue ...
-
ADVERSE REACTIONSSymptoms of anorexia, nausea, and vomiting are the most frequently noted of all toxic reactions. Over 90% of patients are affected with the initial few doses. The vomiting lasts 1 to 12 hours and ...
-
OVERDOSAGEGive supportive treatment and monitor blood cell counts.
-
DOSAGE AND ADMINISTRATIONMalignant Melanoma - The recommended dosage is 2 to 4.5 mg/kg/day for 10 days. Treatment may be repeated at 4 week intervals.2 - An alternate recommended dosage is 250 mg/square meter body ...
-
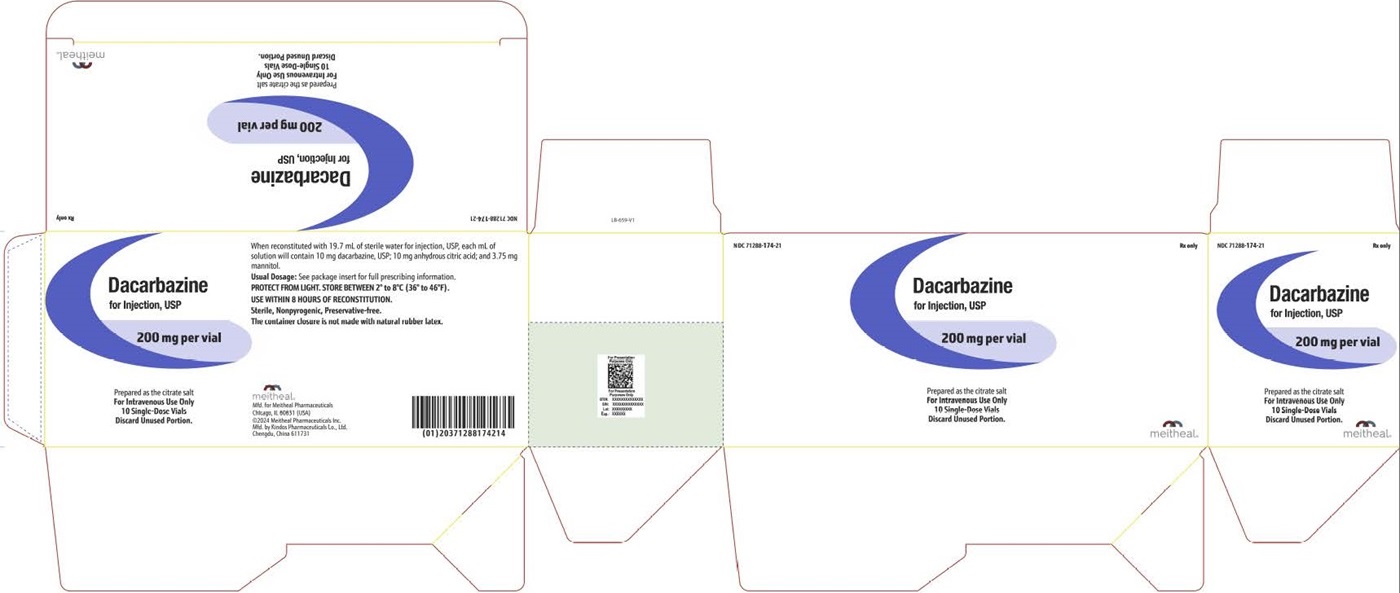
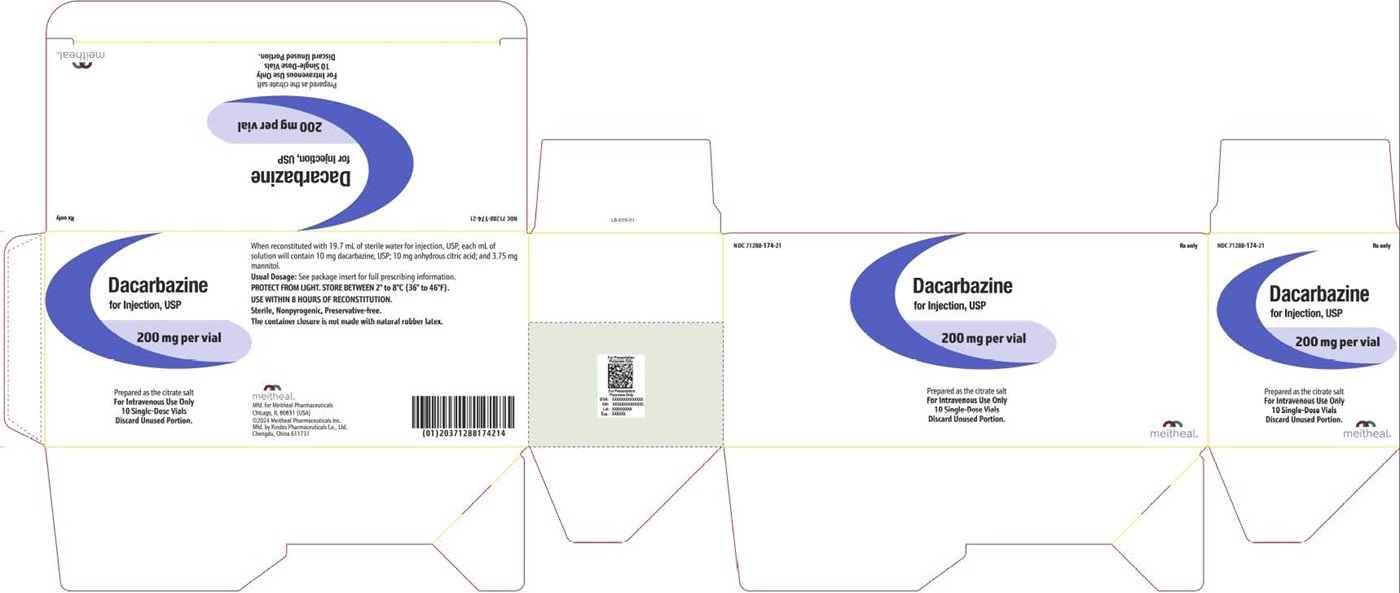
HOW SUPPLIEDSterile Dacarbazine for Injection, USP is available as follows: NDCDacarbazine for Injection, USP Package Factor - 71288-174-21 200 mg Single-Dose Vial 10 vials per carton - Store in ...
-
REFERENCESLoo, T.J., et al.: Mechanism of action and pharmacology studies with DTIC (NSC-45388). Cancer Treatment Reports 60: 149–152, 1976. Nathanson, L., et al.: Characteristics of prognosis and response ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL Dacarbazine for Injection, USP Vial LabelNDC 71288-174-20 - Rx only - Dacarbazine for Injection, USP - 200 mg per vial - Prepared as the citrate salt - For Intravenous Use Only - Single-Dose Vial
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL Dacarbazine for Injection, USP CartonNDC 71288-174-21 - Rx only - Dacarbazine for Injection, USP - 200 mg per vial - Prepared as the citrate salt - For Intravenous Use Only - 10 Single-Dose Vials - Discard Unused Portion.
-
INGREDIENTS AND APPEARANCEProduct Information