Label: TRANEXAMIC ACID IN SODIUM CHLORIDE- tranexamic acid injection, solution
- NDC Code(s): 65219-534-01, 65219-534-10
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRANEXAMIC ACID IN SODIUM CHLORIDE INJECTION safely and effectively. See full prescribing information for TRANEXAMIC ACID IN SODIUM ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Tranexamic Acid in Sodium Chloride Injection is indicated in patients with hemophilia for short-term use (two to eight days) to reduce or prevent hemorrhage and reduce the need for replacement ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosage - The recommended dose of Tranexamic Acid in Sodium Chloride Injection is 10 mg/kg actual body weight intravenously administered as a single dose, immediately before tooth ...
-
3 DOSAGE FORMS AND STRENGTHS Injection: 1,000 mg of tranexamic acid in 100 mL (10 mg/mL), colorless solution in a single-dose bag for intravenous use
-
4 CONTRAINDICATIONS Tranexamic Acid in Sodium Chloride Injection is contraindicated: • In patients with subarachnoid hemorrhage. Anecdotal experience indicates that cerebral edema and cerebral infarction may be ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Thromboembolic Risk - Tranexamic Acid in Sodium Chloride Injection is contraindicated in patients with active intravascular clotting. Tranexamic acid is an antifibrinolytic and may increase ...
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in the labeling: • Thromboembolic Risk [see Warnings and Precautions (5.1)] • Seizures [see Warnings and ...
-
7 DRUG INTERACTIONS 7.1 Prothrombotic Medical Products - Avoid concomitant use of Tranexamic Acid in Sodium Chloride Injection with medical products that are prothrombotic because concomitant use can further ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Available data from published studies, case series and case reports with tranexamic acid use in pregnant women in the second and third trimester and at the time of ...
-
10 OVERDOSAGE Cases of overdosage of tranexamic acid have been reported. Based on these reports, symptoms of overdosage may be gastrointestinal, e.g., nausea, vomiting, diarrhea; hypotensive, e.g., orthostatic ...
-
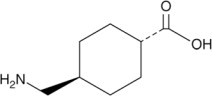
11 DESCRIPTION Tranexamic acid is trans-4-(aminomethyl)cyclohexanecarboxylic acid, an antifibrinolytic agent. Tranexamic acid is a white crystalline powder. The structural formula is: Empirical Formula ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Tranexamic acid is a synthetic lysine amino acid derivative, which diminishes the dissolution of hemostatic fibrin by plasmin. In the presence of tranexamic acid, the ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Tranexamic acid was not carcinogenic in a 2-year study in rats and mice at oral doses up to 3 and 5.3 g/kg/day, which are ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Tranexamic Acid in Sodium Chloride Injection is a sterile, unpreserved, colorless solution in a single-dose polyolefin container with a polypropylene overwrap supplied as: Product ...
-
17 PATIENT COUNSELING INFORMATION Thromboembolic Risk - • Inform patients that Tranexamic Acid in Sodium Chloride Injection may increase the risk of venous and arterial thrombosis or thromboembolism and to contact their ...
-
PACKAGE LABEL – PRINIPAL DISPLAY – Tranexamic Acid in 0.7% Sodium Chloride 100 mL Case Label Product No. 534110 NDC 65219-534-10 - Tranexamic Acid in 0.7% Sodium Chloride Injection - 1,000 mg per 100 mL (10 mg per mL) For Intravenous Infusion Only Rx ...
-
PACKAGE LABEL – PRINIPAL DISPLAY – Tranexamic Acid in 0.7% Sodium Chloride 100 mL Bag Label NDC 65219-534-01 100 mL - Tranexamic Acid - in 0.7% Sodium Chloride Injection - 1,000 mg per 100 mL (10 mg per mL) For Intravenous Infusion Only Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information