Label: VEGETABLE LAXATIVE- sennosides, 8.6 mg tablet, film coated
- NDC Code(s): 63561-0188-1
- Packager: Granulation Technology, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
-
Active ingredients (in each tablet)Sennosides 8.6 mg
-
PurposeLaxative
-
UsesRelieves occasional constipation (irregularity) Generally produces a bowel movement in 6-12 hours
- Warnings
-
Do not useLaxative products for longer than 1 week unless directed by a doctor
-
Ask a doctor before use if you haveStomach pain - Nausea - Vomiting - Noticed a sudden change in bowel habits that continues over a period of 2 weeks
-
Stop using and ask a doctor ifyou have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
-
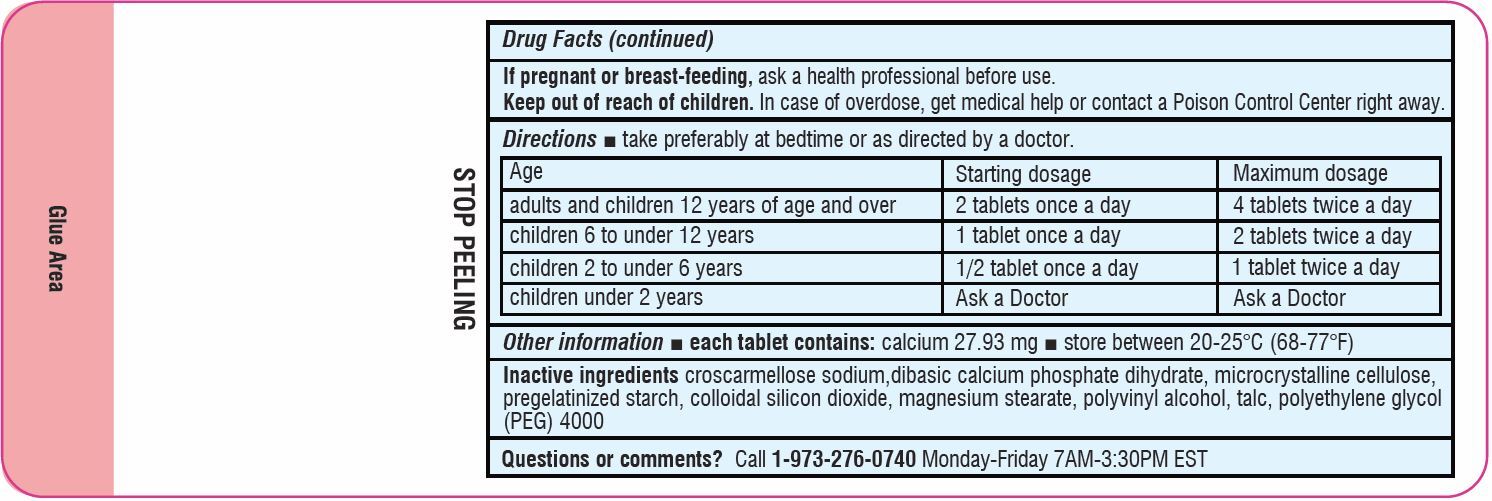
If pregnant or breast-feeding,ask a health professional before use.
-
Keep out of reach of children.In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
-
DirectionsTake preferably at bedtime or as directed by a doctor - agestarting dosagemaximum dosage - adults and children - 12 years of age and over - 2 tablets once a day4 tablets twice a ...
-
Other informationEach tablet contains:Calcium 27.93 mg - Store between 20-25°C (68-77°F)
-
Inactive ingredientscroscarmellose sodium,dibasic calcium phosphate dihydrate, microcrystalline cellulose, pregelatinized starch, colloidal silicon dioxide, magnesium stearate, polyvinyl alcohol, polyethylene glycol ...
-
Principal Display PanelNDC 63561-0188-1 - Vegetable Laxative Label - SENNOSIDES, 8.6 mg LAXATIVE - Compare to Senokot - ® Regular Strength Active Ingredients - 100 Film-Coated Tablets
-
INGREDIENTS AND APPEARANCEProduct Information