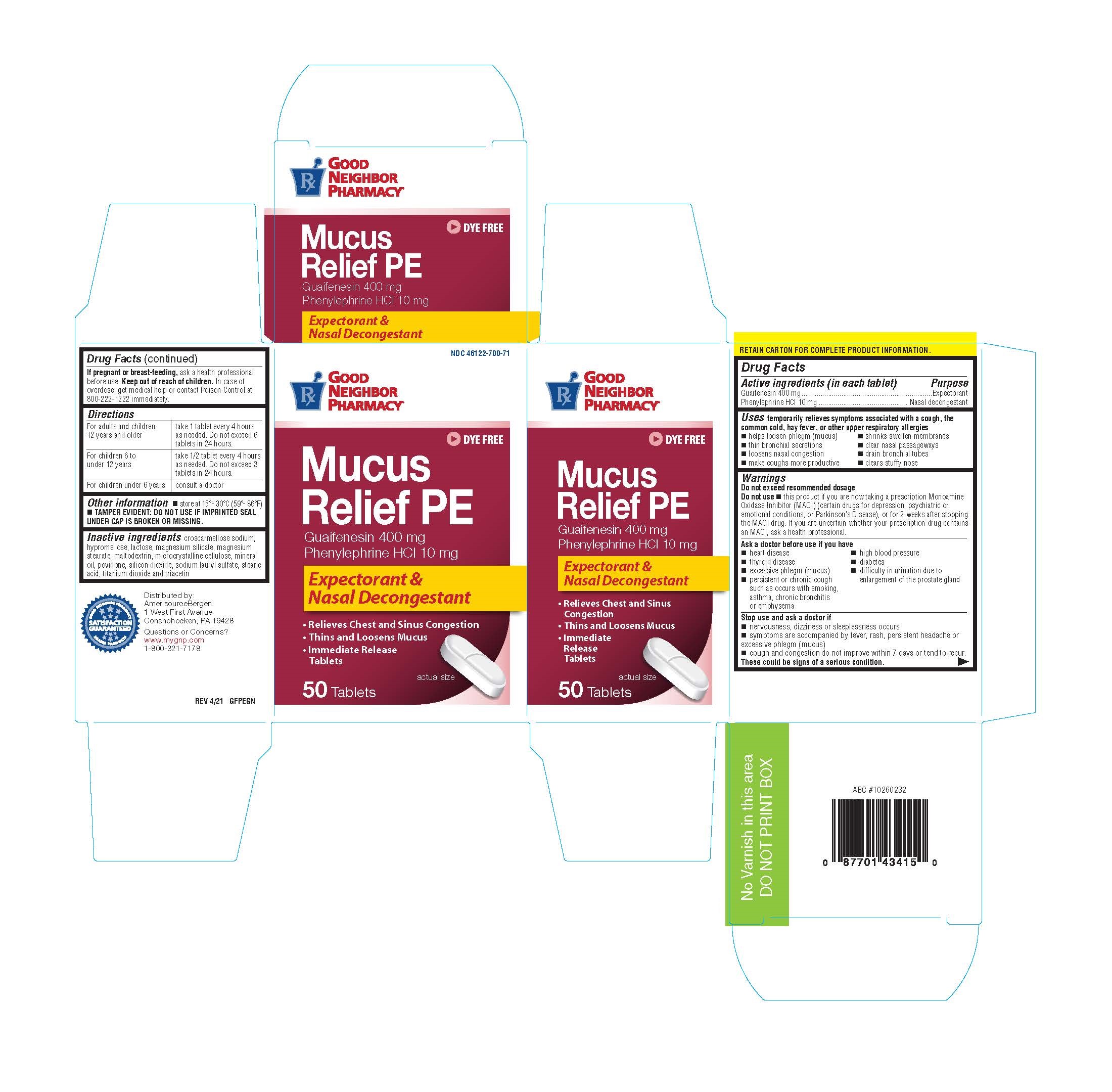
Label: GNP MUCUS RELIEF PE- guaifenesin/phenylephrine tablet
- NDC Code(s): 46122-700-71
- Packager: AmerisourceBergen Drug Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
-
Uses
Temporarily relieves symptoms associated with a cough ,the common cold,hay fever or other upper respiratory allergies.
- helps loosen phlegm (mucus)
- clear nasal passageways
- loosens nasal congestion
- drain bronchial tubes
- shrinks swollen membranes
- clears stuffy nose
- makes coughs more productive
- thin bronchial secretions
- Warnings
-
Do not use
This product if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression,
psychiatric or emotional conditions, or Parkinson’s Disease), or for 2 weeks after stopping the
MAOI drug. If you are uncertain whether your prescription drug contains an MAOI, ask a health
professional. - Ask a doctor before use if you have
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive ingredients
- Carton Panel
-
INGREDIENTS AND APPEARANCE
GNP MUCUS RELIEF PE
guaifenesin/phenylephrine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-700 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Guaifenesin (UNII: 495W7451VQ) (Guaifenesin - UNII:495W7451VQ) Guaifenesin 400 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) POVIDONE (UNII: FZ989GH94E) MAGNESIUM SILICATE (UNII: 9B9691B2N9) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white Score 2 pieces Shape OVAL Size 18mm Flavor Imprint Code PH043 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-700-71 1 in 1 CARTON 01/13/2022 1 50 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/13/2022 Labeler - AmerisourceBergen Drug Corp (007914906) Registrant - Reese Pharmaceutical Co (004172052) Establishment Name Address ID/FEI Business Operations Pharbest 557054835 manufacture(46122-700)