Label: NOREPINEPHRINE BITARTRATE injection, solution
- NDC Code(s): 0338-0108-20, 0338-0112-20, 0338-0116-20
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NOREPINEPHRINE BITARTRATE IN DEXTROSE injection safely and effectively. See full prescribing information for NOREPINEPHRINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Norepinephrine Bitartrate in Dextrose Injection is indicated to raise blood pressure in adult patients with severe, acute hypotension.
-
2 DOSAGE AND ADMINISTRATION 2.1 Important Dosage and Administration Instructions - Correct Hypovolemia - Address hypovolemia before initiation of Norepinephrine Bitartrate in Dextrose Injection therapy. If the patient does ...
-
3 DOSAGE FORMS AND STRENGTHS Injection: Norepinephrine bitartrate in 5% dextrose is a colorless to slightly yellow solution for intravenous infusion, supplied in 250-mL single dose containers as: • 4 mg equivalent of ...
-
4 CONTRAINDICATIONS None.
-
5 WARNINGS AND PRECAUTIONS 5.1 Tissue Ischemia - Administration of Norepinephrine Bitartrate in Dextrose Injection to patients who are hypotensive from hypovolemia can result in severe peripheral and visceral ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are described in greater detail in other sections: • Tissue Ischemia [see Warnings and Precautions (5.1)] • Hypotension [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS 7.1 MAO-Inhibiting Drugs - Co-administration of Norepinephrine Bitartrate in Dextrose Injection with monoamine oxidase (MAO) inhibitors or other drugs with MAO-inhibiting properties (e.g. ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Limited published data consisting of a small number of case reports and multiple small trials involving the use of norepinephrine in pregnant women at the time of ...
-
10 OVERDOSAGE Overdosage with Norepinephrine Bitartrate in Dextrose Injection may result in headache, severe hypertension, reflex bradycardia, marked increase in peripheral resistance, and decreased cardiac ...
-
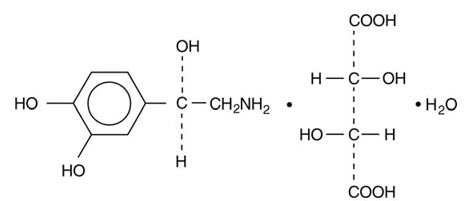
11 DESCRIPTION Norepinephrine Bitartrate in Dextrose Injection contains norepinephrine, a sympathomimetic amine. Norepinephrine is sometimes referred to as l-arterenol/Levarterenol or l-norepinephrine which ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Norepinephrine is a peripheral vasoconstrictor (alpha-adrenergic action) and an inotropic stimulator of the heart and dilator of coronary arteries (beta-adrenergic ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis, mutagenesis, and fertility studies have not been performed.
-
16 HOW SUPPLIED/STORAGE AND HANDLING Norepinephrine Bitartrate in Dextrose Injection for intravenous infusion is a colorless to slightly yellow solution available in single-dose, ready-to-use containers in an amber/foil overwrap ...
-
17 PATIENT COUNSELING INFORMATION Risk of Tissue Damage - Advise the patient, family, or caregiver to report signs of extravasation urgently [see Warnings and Precautions (5.1)].
-
SPL UNCLASSIFIED SECTIONManufactured by: Baxter Healthcare Corporation - Deerfield, IL 60015 USA - Made in Ireland - Baxter and Viaflo are trademarks of Baxter International, Inc or its subsidiaries. CB-30-02-920
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL Container Label - EZPE7748 - NDC 0338-0112-20 - 250 mL - Norepinephrine - Bitartrate in 5% Dextrose Injection - 4 mg / 250 mL - (16 mg / mL) For Intravenous Infusion Only - Each 100mL of sterile ...
-
INGREDIENTS AND APPEARANCEProduct Information