Label: SODIUM CITRATE AND CITRIC ACID- sodium citrate and citric acid monohydrate solution
-
NDC Code(s):
0121-0595-00,
0121-0595-15,
0121-0595-16,
0121-0595-30, view more0121-1190-00, 0121-1190-30
- Packager: Pharmaceutical Associates, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Sodium Citrate and Citric Acid Oral Solution USP is a stable and pleasant-tasting systemic alkalizer containing sodium citrate and citric acid in a sugar-free base. It is a nonparticulate neutralizing buffer.
Sodium Citrate and Citric Acid Oral Solution USP contains in each teaspoonful (5 mL):
SODIUM CITRATE Dihydrate 500 mg (0.34 Molar)
CITRIC ACID Monohydrate 334 mg (0.32 Molar)Each mL contains 1 mEq sodium ion and is equivalent to 1 mEq bicarbonate (HCO3).
Sodium citrate contains the following inactive ingredients: flavoring, polyethylene glycol, propylene glycol, purified water, sodium benzoate, and sorbitol solution.
-
CLINICAL PHARMACOLOGY
Sodium citrate is absorbed and metabolized to sodium bicarbonate, thus acting as a systemic alkalizer. The effects are essentially those of chlorides before absorption and those of bicarbonates subsequently. Oxidation is virtually complete so that less than 5% of sodium citrate is excreted in the urine unchanged.
-
INDICATIONS AND USAGE
Sodium Citrate and Citric Acid Oral Solution USP is an effective alkalinizing agent. It is useful in those conditions where long-term maintenance of an alkaline urine is desirable, and is of value in the alleviation of chronic metabolic acidosis, such as results from chronic renal insufficiency or the syndrome of renal tubular acidosis, especially when the administration of potassium salts is undesirable or contraindicated. This product is also useful for buffering and neutralizing gastric hydrochloric acid quickly and effectively.
Sodium Citrate and Citric Acid Oral Solution USP is concentrated, and when administered after meals and before bedtime, allows one to maintain an alkaline urinary pH around the clock, usually without the necessity of a 2 A.M. dose. This product alkalinizes the urine without producing a systemic alkalosis in the recommended dosage. This product is highly palatable, pleasant tasting, and tolerable, even when administered for long periods.
- CONTRAINDICATIONS
-
PRECAUTIONS
Sodium Citrate and Citric Acid Oral Solution USP should be used with caution by patients with low urinary output unless under the supervision of a physician. This product should not be administered concurrently with aluminum-based antacids. Patients should be directed to dilute adequately with water and preferably, to take each dose after meals to avoid saline laxative effect. Sodium salts should be used cautiously in patients with cardiac failure, hypertension, impaired renal function, peripheral and pulmonary edema, and toxemia of pregnancy. Periodic examinations and determinations of serum electrolytes, particularly serum bicarbonate level, should be carried out in those patients with renal disease in order to avoid these complications.
-
ADVERSE REACTIONS
Sodium Citrate and Citric Acid Oral Solution USP is generally well tolerated, without any unpleasant side effects, when given in recommended doses to patients with normal renal function and urinary output. However, as with any alkalinizing agent, caution must be used in certain patients with abnormal renal mechanisms to avoid development of alkalosis, especially in the presence of hypocalcemia.
To report SUSPECTED ADVERSE REACTIONS, contact Pharmaceutical Associates, Inc. at 1-800-845-8210 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch..
- OVERDOSAGE
- DOSAGE AND ADMINISTRATION
- For Systemic Alkalization
- As a neutralizing buffer
-
HOW SUPPLIED
Sodium Citrate and Citric Acid Oral Solution USP (colorless, grape flavor) is supplied in the following oral dosage forms:
NDC 0121-0595-16: 16 fl oz (473 mL) bottle
NDC 0121-0595-15: 15 mL unit dose cup. Case contains 100 unit-dose cups of 15 mL (NDC 0121-0595-00), packaged in 10 trays of 10 unit-dose cups each.
NDC 0121-1190-30: 30 mL unit dose cup. Case contains 100 unit-dose cups of 30 mL (NDC 0121-1190-00) packaged in 10 trays of 10 unit-dose cups each.
.
- STORAGE:
- SPL UNCLASSIFIED SECTION
-
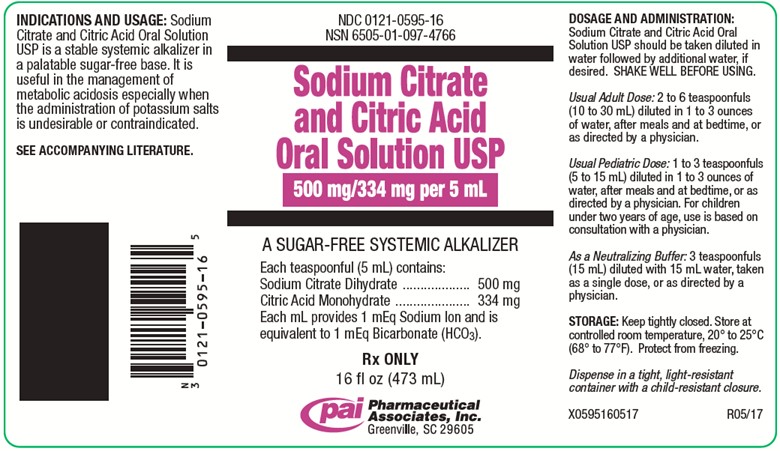
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
NDC 0121-0595-16
NSN 6505-01-097-4766Sodium Citrate and Citric Acid Oral Solution USP
500 mg/334 mg per 5 mL
A SUGAR-FREE SYSTEMIC ALKALIZER
Each teaspoonful (5 mL) contains:
Sodium Citrate Dihydrate..........500 mg
Citric Acid Monohydrate............334 mg
Each mL provides 1 mEq Sodium Ion and is
equivalent to 1 mEq Bicarbonate (HCO3).Rx ONLY
16 fl oz (473 mL)
Pharmaceutical Associates, Inc.
Greenville, SC 29605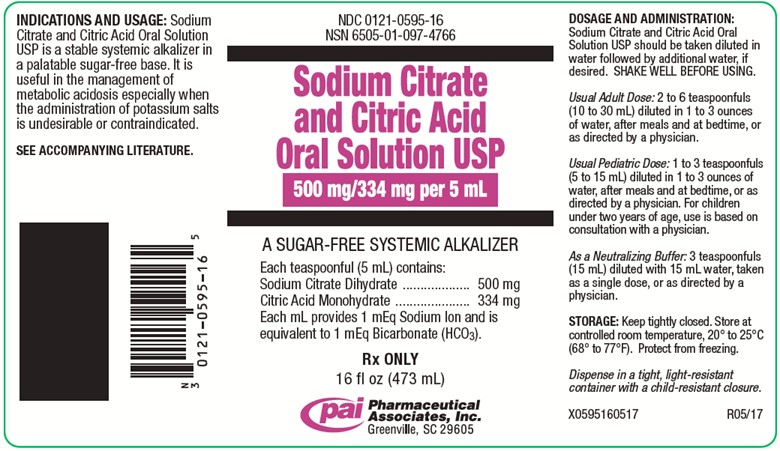
- PRINCIPAL DISPLAY PANEL - 15 mL Unit Dose Cup Label
- PRINCIPAL DISPLAY PANEL - 30 mL Unit Dose Cup Label
-
INGREDIENTS AND APPEARANCE
SODIUM CITRATE AND CITRIC ACID
sodium citrate and citric acid monohydrate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0121-0595 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) SODIUM CITRATE 500 mg in 5 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 334 mg in 5 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0121-0595-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/1969 2 NDC:0121-0595-00 10 in 1 CASE 01/01/1969 2 10 in 1 TRAY 2 NDC:0121-0595-15 15 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 3 NDC:0121-0595-30 10 in 1 CASE 01/01/1969 3 10 in 1 TRAY 3 30 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1969 SODIUM CITRATE AND CITRIC ACID
sodium citrate and citric acid monohydrate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0121-1190 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) SODIUM CITRATE 500 mg in 5 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) ANHYDROUS CITRIC ACID 334 mg in 5 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0121-1190-00 10 in 1 CASE 01/01/1969 1 10 in 1 TRAY 1 NDC:0121-1190-30 30 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1969 Labeler - Pharmaceutical Associates, Inc. (044940096) Establishment Name Address ID/FEI Business Operations Pharmaceutical Associates, Inc. 097630693 manufacture(0121-0595, 0121-1190)