Label: GARNIER OMBRELLE SPORT 30- avobenzone, octisalate, octocrylene and oxybenzone spray
- NDC Code(s): 49967-285-01
- Packager: L'OREAL USA PRODUCTS INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
-
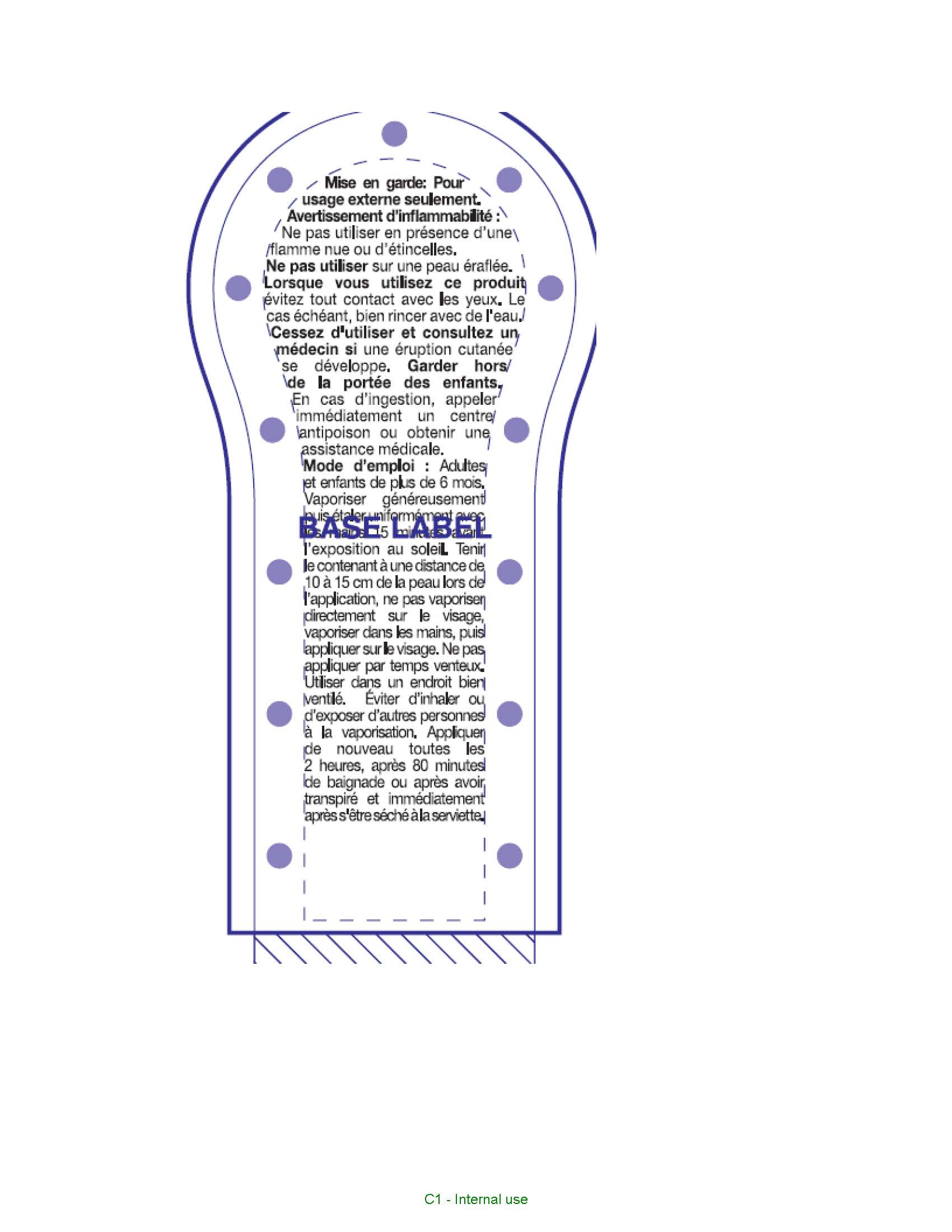
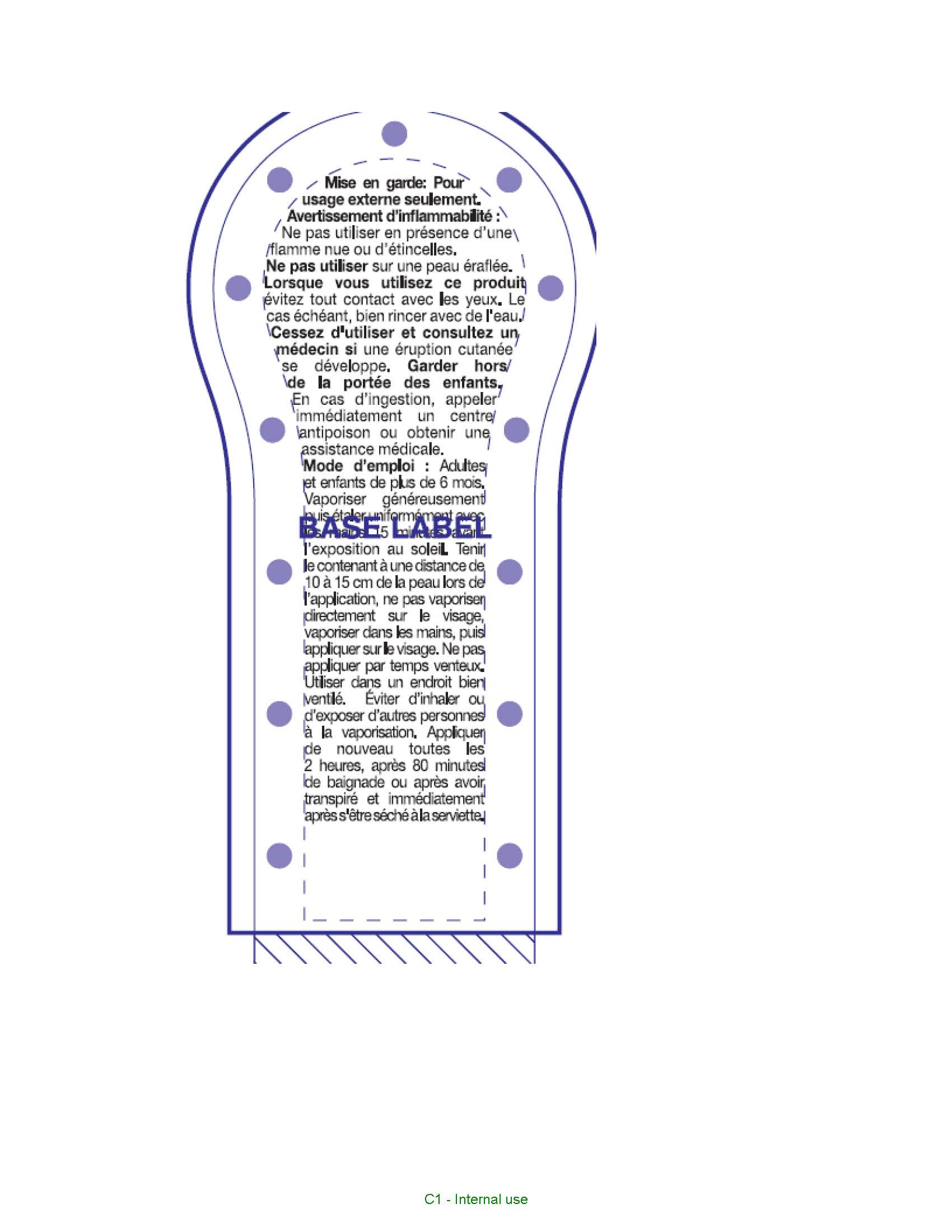
Warnings
For external use only. Flammability Warning. Do not use in presence of open flame or spark. When using this product avoid contact witheyes. If contact occurs, rinse thoroughlly with water. Do not use on broken skin. Stop use and ask a doctor if rash occurs. Other warnings. Contents under pressure, do not place in hot water or near radiator, stoves or other sources of heat, do not puncture or incinerate container or store at temperatures over 50oC. Keep out of reach of children. If swallowed get medical help or contact a poison control center right away.
-
Directions
Adults and children over 6 months of age. Shake well before use. Spray generously and spread evenly by hand 15 minutes before sun exposure. Hold container 10 to 15 cm from the skin to apply, do not spray directly onto face. Spray on hand then apply to face. Do not apply in windy conditions and use in a well ventilated area, avoid inhaling or exposing others to spray. Reapply at least every 2 hours, after 80 minutes of swimming or sweating, right after towel drying.
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GARNIER OMBRELLE SPORT 30
avobenzone, octisalate, octocrylene and oxybenzone sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-285 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-285-01 145 mL in 1 CONTAINER; Type 0: Not a Combination Product 03/27/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date export only 03/27/2007 Labeler - L'OREAL USA PRODUCTS INC (002136794) Establishment Name Address ID/FEI Business Operations L'OREAL USA, INC 624244349 manufacture(49967-285) , pack(49967-285)