Label: MUCUS RELIEF DM COUGH MAXIMUM STRENGTH- dextromethorphan hbr and guaifenesin tablet, film coated
- NDC Code(s): 70000-0278-1, 70000-0278-2
- Packager: Cardinal Health 110, LLC. DBA Leader
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 8, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
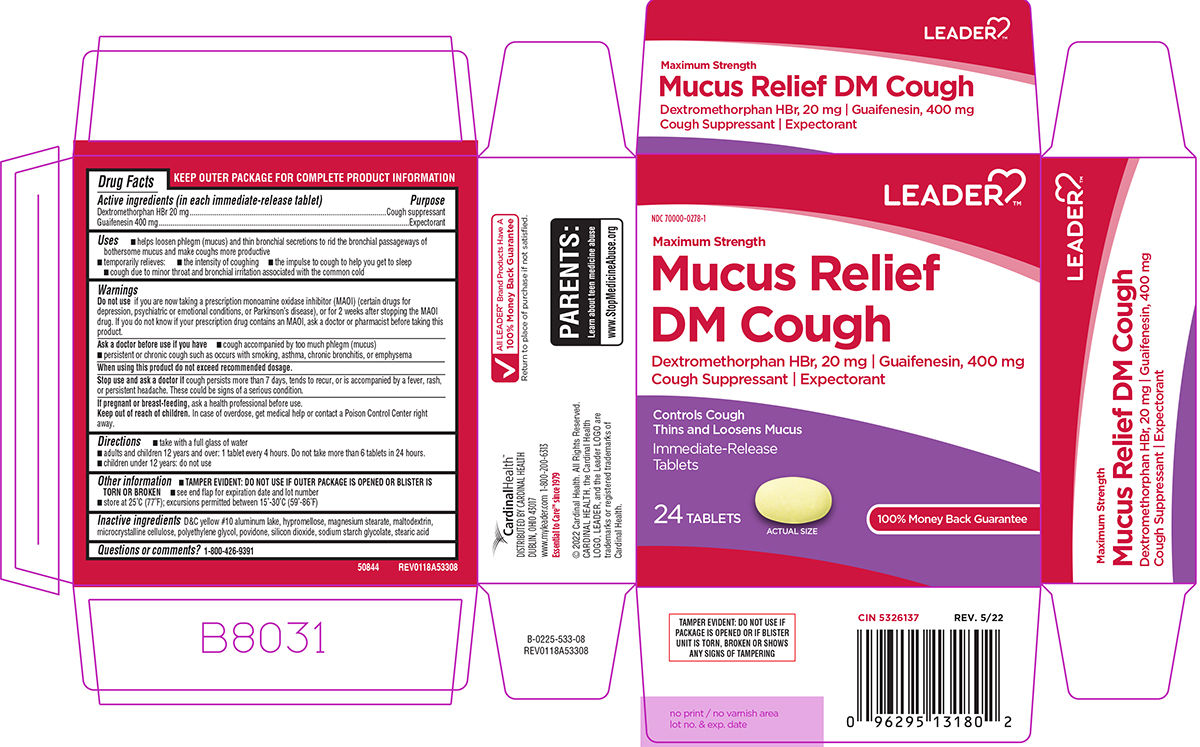
Active ingredients (in each immediate-release tablet)
Dextromethorphan HBr 20 mg - Guaifenesin 400 mg
-
Purpose
Cough suppressant - Expectorant
-
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive - temporarily relieves: cough due to minor throat ...
-
Warnings
Do not use - if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks ...
-
Directions
take with a full glass of water - adults and children 12 years and over: 1 tablet every 4 hours. Do not take more than 6 tablets in 24 hours. children under 12 years: do not use
-
Other information
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN - see end flap for expiration date and lot number - store at 25°C (77°F); excursions permitted between ...
-
Inactive ingredients
D&C yellow #10 aluminum lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, povidone, silicon dioxide, sodium starch glycolate, stearic ...
-
Questions or comments?
1-800-426-9391
-
Principal display panel
LEADER™ NDC 70000-0278-1 - Maximum Strength - Mucus Relief - DM Cough - Dextromethorphan HBr, 20 mg ι Guaifenesin, 400 mg - Cough Suppressant ι Expectorant - Controls Cough - Thins and Loosens ...
-
INGREDIENTS AND APPEARANCEProduct Information