Label: MEGATOPE- iodinated i-131 albumin injection, solution
- NDC Code(s): 50914-7731-4
- Packager: Iso-Tex Diagnostics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated September 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only.
-
DESCRIPTIONChemical Characteristics - MEGATOPE (iodinated I 131 albumin) injection is a sterile, nonpyrogenic, radioactive diagnostic agent for intravenous use. Each mL contains albumin human (approximately ...
-
CLINICAL PHARMACOLOGY
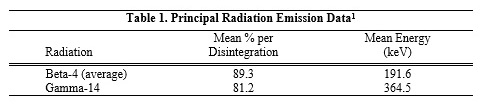
Following intravenous injection, iodinated I 131 albumin is distributed throughout the intravascular pool within 10 minutes; extravascular distribution takes place more slowly. Iodinated I 131 ...
-
INDICATIONS AND USAGE
MEGATOPE is indicated in adults for use in determinations of total blood and plasma volumes.
-
CONTRAINDICATIONS
None Known.
-
WARNINGS
Aseptic meningitis and pyrogenic reactions have been reported following cisternography with MEGATOPE. The safety and effectiveness of MEGATOPE for cisternography have not been established ...
-
PRECAUTIONS
General - In the use of any radioactive material, care should be taken to minimize radiation exposure to the patient and healthcare providers consistent with proper patient ...
-
ADVERSE REACTIONS
The following adverse reactions have been identified with the use of radioiodinated albumin products. Because these reactions are reported voluntarily from a population of uncertain size, it is ...
-
DOSAGE AND ADMINISTRATION
Premedication - Administer 10 drops of Strong Iodine Solution, USP (e.g., Lugol’s Solution) three times daily, beginning at least 24 hours before administration of MEGATOPE and continue for 1 week ...
-
HOW SUPPLIED
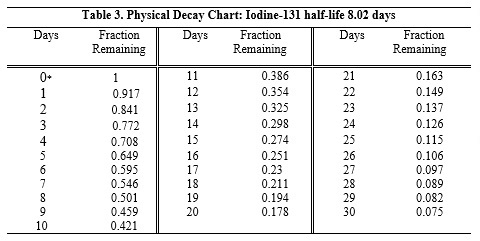
MEGATOPE (iodinated I 131 albumin) injection is a colorless to very pale yellow solution available in multiple-dose vials containing the following amounts of radioactivity on the date of ...
-
STORAGE AND HANDLINGStore refrigerated at 2º to 8ºC (36° to 46°F) in suitable lead shield.
-
SPL UNCLASSIFIED SECTIONThis radiopharmaceutical is licensed for distribution to facilities and persons licensed by the U.S. Nuclear Regulatory Commission or under an equivalent license issued by an Agreement ...
-
Packaging

-
INGREDIENTS AND APPEARANCEProduct Information