Label: EOVIST- gadoxetate disodium injection, solution
- NDC Code(s): 50419-320-05, 50419-320-15, 50419-320-75
- Packager: Bayer HealthCare Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 5, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EOVIST® safely and effectively. See full prescribing information for EOVIST. EOVIST (gadoxetate disodium) injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS (NSF)
Risk Associated with Intrathecal Use
Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. EOVIST is not approved for intrathecal use [see Warnings and Precautions (5.1)]
Nephrogenic Systemic Fibrosis
GBCAs increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of EOVIST in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.
The risk for NSF appears highest among patients with:
-
- o
- Chronic, severe kidney disease (GFR < 30 mL/min/1.73m2), or
- o
- Acute kidney injury.
Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (for example, age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
For patients at highest risk for NSF, do not exceed the recommended EOVIST dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration [see Warnings and Precautions (5.2)].
Close -
1 INDICATIONS AND USAGEEOVIST is indicated for intravenous use in magnetic resonance imaging (MRI) of the liver to detect and characterize lesions in patients with known or suspected focal liver disease.
-
2.1 Recommended DoseThe recommended dose of EOVIST is 0.1 mL/kg body weight (0.025 mmol/kg body weight).
-
DOSAGE & ADMINISTRATION2.2 Drug Handling and Administration - • Use sterile technique when preparing and administering EOVIST - • Visually inspect EOVIST, supplied in a single-dose container (vial), for particulate ...
-
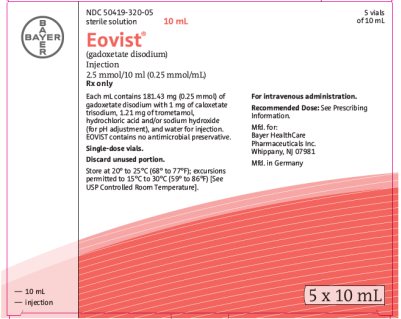
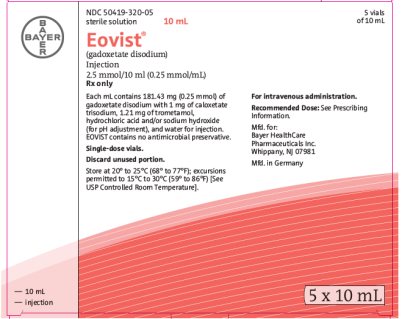
3 DOSAGE FORMS AND STRENGTHSEOVIST is a sterile, clear, and colorless to pale yellow solution for injection containing 181.43 mg gadoxetate disodium per mL (equivalent to 0.25 mmol gadoxetate disodium per mL) supplied in ...
-
4 CONTRAINDICATIONSEOVIST is contraindicated in patients with history of severe hypersensitivity reactions to EOVIST [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Risk Associated with Intrathecal Use - Intrathecal administration of GBCAs can cause serious adverse reactions including death, coma, encephalopathy, and seizures. The safety and ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed elsewhere in the labeling: • Nephrogenic systemic fibrosis (NSF) [see Boxed Warning and Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - GBCAs have been shown to cross the human placenta and result in fetal exposure and gadolinium retention. The human data on the association between GBCAs and ...
-
10 OVERDOSAGEThe maximum dose studied in MR imaging was 0.4 mL/kg (0.1 mmol/kg) body weight and was tolerated in a manner similar to lower doses. In case of inadvertent overdosage in patients with severely ...
-
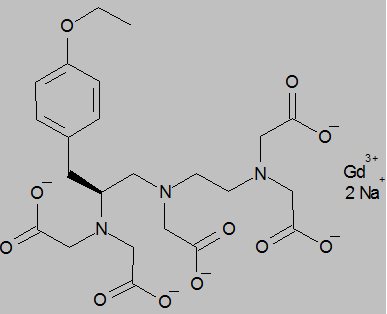
11 DESCRIPTIONEOVIST (gadoxetate disodium) is a paramagnetic contrast agent administered for MRI. EOVIST is provided as a sterile, clear, colorless to pale yellow aqueous solution for intravenous ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Gadoxetate disodium is a paramagnetic compound and develops a magnetic moment when placed in a magnetic field. The relatively large magnetic moment produced by ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity studies of EOVIST have been conducted. Gadoxetate disodium was not mutagenic in in vitro reverse mutation tests in ...
-
14 CLINICAL STUDIESPatients with suspected or known focal liver lesions were enrolled in two of four non-randomized, intrapatient-controlled studies that evaluated predominantly the detection (studies 1 and 2) or ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - EOVIST is supplied in single-dose, rubber stoppered containers (vials) containing 181.43 mg/mL of gadoxetate disodium (equivalent to 0.25 mmol/mL gadoxetate disodium), in the ...
-
17 PATIENT COUNSELING INFORMATION• Advise the patient to read the FDA-approved patient labeling (Medication Guide). Nephrogenic Systemic Fibrosis - Instruct patients to inform their physician if they: • Have a history of ...
-
MEDICATION GUIDEMEDICATION GUIDE - EOVIST - (e-o-vist) (gadoxetate disodium) Injection for intravenous use - What is the most important information I should know about Eovist? • GBCAs like EOVIST may ...
-
Carton 5 x 10 mL NDC 50419-320-05 - 5 vials of 10 mL - sterile solution - Eovist® 10 mL - (gadoxetate disodium) Injection - 0.25 mol/L - Rx only - Each mL contains 181.43 gadoxetate disodium and the excipients caloxetate ...
-
INGREDIENTS AND APPEARANCEProduct Information