Label: ETHIQA XR- buprenorphine hydrochloride injection, suspension, extended release
- NDC Code(s): 86084-100-30
- Packager: Fidelis Animal Health, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: Legally Marketed Unapproved New Animal Drugs for Minor Species
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
LEGAL STATUS--In order to be legally marketed, a new animal drug intended for a minor species must be Approved, Conditionally Approved, or Indexed by the Food and Drug Administration. THIS PRODUCT IS INDEXED--MIF 900-014. Extra-label use is prohibited.
This product is not to be used in animals intended for use as food for humans or food-producing animals.
-
ABUSE
HUMAN SAFETY WARNING
Abuse Potential
ETHIQA XR contains buprenorphine, an opioid that exposes humans to risks of misuse, abuse, and addiction, which can lead to overdose and death. Use of buprenorphine may lead to physical dependence. The risk of abuse by humans should be considered when storing, administering, and disposing of ETHIQA XR. Persons at increased risk for opioid abuse include those with a personal or family history of substance abuse (including drugs or alcohol) or mental illness (e.g., depression).Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression may occur with accidental exposure to or with misuse or abuse of ETHIQA XR. Monitor for respiratory depression if human exposure to buprenorphine occurs. Misuse or abuse of buprenorphine by swallowing, snorting, or injecting poses a significant risk of overdose and death.Accidental Exposure
Because of the potential for adverse reactions associated with accidental exposure, ETHIQA XR should only be administered by veterinarians, veterinary technicians, or laboratory staff who are trained in the handling of potent opioids. Accidental exposure to ETHIQA XR, especially in children, can result in a fatal overdose of buprenorphine.Risks From Concurrent Misuse or Abuse with Benzodiazepines or Other CNS Depressants
Concurrent misuse or abuse of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death.See HUMAN SAFETY WARNINGS for detailed information.
-
DESCRIPTION
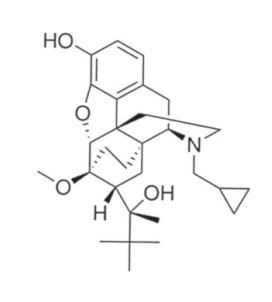
Ethiqa XR is an injectable suspension of extended-release buprenorphine. Ethiqa XR is an extended-release formulation using the Fidelipid LAI™ technology, a proprietary lipid combination of glycerides and cholesterol. Buprenorphine hydrochloride, an opioid analgesic, is the active ingredient in Ethiqa XR. Lipid-bound buprenorphine hydrochloride is suspended in medium chain fatty acid triglyceride (MCT) oil. Lipids encapsulate the buprenorphine limiting diffusion which provides for larger doses and prolonged action.1,2 Ethiqa XR has a slightly yellow to white opaque appearance. Each mL contains approximately 1.3 mg buprenorphine hydrochloride. The sterile product contains cholesterol, benzyl alcohol, glyceryl tristearate, and buprenorphine hydrochloride suspended in MCT oil. Buprenorphine belongs to the opioid class of drugs and is a narcotic under the Controlled Substances Act due to its chemical derivation from thebaine.
Buprenorphine
Formula C29H41NO4
- INDICATIONS
-
DOSAGE AND ADMINISTRATION
Wear protective clothing when administering Ethiqa XR.
Do not dispense Ethiqa XR for administration at home by the pet owner (see HUMAN SAFETY WARNINGS).
Dosing
Administer Ethiqa XR subcutaneously according to the dose listed in the table for the appropriate species.Doses were derived either from published literature or using allometric principles.
Consider the time to reach estimated therapeutic blood levels when administering Ethiqa XR for post-procedural pain. If needed, a single repeat dose may be administered subcutaneously 72 hours after the initial dose.
Definitive therapeutic blood levels of Ethiqa XR have not been established for all species. The times to reach blood levels thought to be therapeutic is presented below and is representative of what has been found in published literature.
For more information, consult the published literature referenced at the end of this package insert.
DOSING TABLE FOR SUBCUTANEOUS INJECTION OF ETHIQA XR
Species Ethiqa XR
Dose (mg/kg
body weight)Time to reach
estimated
therapeutic
blood levelsPrecautions/
Adverse EventsMice 3.25 mg/kg10 30 minutes10 • Death has been reported when non-steroidal anti-inflammatory drugs (NSAIDs such as meloxicam and carprofen) and Ethiqa XR
have been administered
concomitantly.5
• Granulomatous inflammatory
nodules have been observed
in naked-skinned mice and rats administered Ethiqa XR.4,5
• In one study, two male mice died following the third surgery and redosing; weight loss.11Naked
mole rats
(NMR)3.25 mg/kg* • No published data available administering Ethiqa XR to naked mole rats. Gerbils 1 mg/kg13 30 minutes13 • Granulomatous inflammation at injection site.13 Hamsters 0.8 mg/kg* • No published data available administering Ethiqa XR to hamsters. Rats 0.65 mg/kg12 4 hours16 • Nausea within 24 hrs of dosing, self-licking, self-gnawing and efforts to eat wood-chip bedding, one out of 36 rats exposed to
wood bedding died3,12, 3 of
222 rats bled profusely from jugular vein, which was used for obtaining blood samples, and died.
• Granulomatous inflammatory
nodules have been observed
in naked-skinned mice and
rats administered Ethiqa XR.4,5Chinchillas 0.48 mg/kg* • No published data available administering Ethiqa XR to chinchillas. Guinea pigs 0.48 mg/kg17* 8 hours17 • Decrease in body weight14,17and fecal output.14 Increase in passive behavior, such as eyes closed or squinting, subtle body movement, and
incomplete movement.17Prairie dogs 0.48 mg/kg* • No published data available administering Ethiqa XR to prairie dogs. Ferrets 0.6 mg/kg9 30 minutes9 • No adverse reactions observed.9 Non-human
primates0.2 mg/kg6 15 minutes6 • Injection site reactions including inflammation and necrosis have been observed in common marmosets.6
• Mild sedation, decreased body weight, increased cage movements, acute necrosis and inflammation at the injection site.6Laboratory
rabbits0.15 mg/kg19 • 60 minutes19
• 30 minutes in
female and
60 minutes in
male rabbits20• Reduced fecal output post-operatively. Returned to normal at 72 hours.19 *These doses are based on allometric principles.
Allometric principles (i.e., animals among closely related species and of similar body size should have similar metabolic rates) can be used to determine the dose of Ethiqa XR for rodent species not listed in the table above and where no published data is available.
For example, doses for hamsters and guinea pigs were calculated using published allometric scaling factors (see Nair21 and FDA22 for detailed discussion and how to apply allometric scaling).
The dose of Ethiqa XR can also be estimated by using the known dose for a rodent species of similar size (the doses listed in the table above for NMR, chinchillas, and prairie dogs were calculated using this approach).
Based upon the time to reach estimated therapeutic blood levels, Ethiqa XR can be administered 30 minutes prior to painful stimulus in mice10 and gerbils13, 8-12 hours prior in guinea pigs17, 60 minutes prior in laboratory rabbits19, and 15 minutes prior in non-human primates.6
Administration
Shake the vial well before each use to ensure uniform suspension. If stored refrigerated, bring to room temperature before use.
Use aseptic technique to subcutaneously administer Ethiqa XR by utilizing minimally stressful restraint techniques or sedation.
An oily sheen may be observed in the fur after injection due to leakage of Ethiqa XR, which is an oil-based drug suspension, from the injection site. The oily sheen may last for 4 to 5 days post-injection. Leakage from the injection site can be minimized by slowly injecting Ethiqa XR into the subcutaneous space.
Do not return any unused drug suspension from the syringe back into the vial.
The animal can be returned to its cage immediately after receiving Ethiqa XR. (See CONTRAINDICATIONS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION for additional information on bedding.)
-
CONTRAINDICATIONS
Only administer Ethiqa XR by subcutaneous injection. Ethiqa XR is not intended for intravenous, intra-arterial, intrathecal, intramuscular, or intra-peritoneal injection.
Do not use in animals with pre-existing respiratory compromise.
Do not house rats on wood chip-type bedding after administration of Ethiqa XR. Signs of nausea, including pica, have been observed in rats for up to 3 days post-treatment with Ethiqa XR. Pica involving wood chip type bedding can be lethal (see ADVERSE REACTIONS).
-
HUMAN SAFETY WARNINGS
Not for use in humans. Keep this and all medications out of reach of children and pets.Human User Safety While Handling Ethiqa XR in the Hospital:
Ethiqa XR should only be handled and administered by a veterinarian, veterinary technician, or laboratory staff trained in the handling of potent opioids.To prevent human adverse reactions or abuse, at least 2 trained administrators should be present during injection of Ethiqa XR.
Wear protective clothing when administering Ethiqa XR.
Mucous Membrane or Eye Contact During Application:
Direct contact of Ethiqa XR with the eyes, oral, or other mucous membranes could result in absorption of buprenorphine and the potential for adverse reactions. If accidental eye, oral, or other mucous membrane contact is made during application, flush the area with water and contact a physician immediately. If wearing contact lenses, flush the eye first and then remove the contact lens.Skin Contact During Application:
If human skin is accidentally exposed to ETHIQA XR, wash the exposed area immediately with soap and water and contact a physician. Accidental exposure could result in absorption of buprenorphine and the potential for adverse reactions.Drug Abuse, Addiction, and Diversion of Opioids:
Controlled Substance:
Ethiqa XR contains buprenorphine, a Schedule III controlled substance with an abuse potential similar to other Schedule III opioids.Abuse:
Ethiqa XR contains buprenorphine, an opioid substance, that can be abused and is subject to misuse, abuse, and addiction, which may lead to overdose and death. This risk is increased with concurrent use of alcohol and other central nervous system depressants, including other opioids and benzodiazepines.Ethiqa XR should be handled appropriately to minimize the risk of diversion, including restriction of access, the use of accounting procedures, and proper disposal methods, as appropriate to the clinical setting and as required by law.
Prescription drug abuse is the intentional, non-therapeutic use of a prescription drug, even once, for its rewarding psychological or physiological effects. Buprenorphine has been diverted for non-medical use into illicit channels of distribution. All people handling opioids require careful monitoring for signs of abuse.
Storage and Disposal:
Ethiqa XR is a Schedule III opioid. Store in a locked cabinet according to federal and state controlled substance requirements/guidelines. Discard any broached vials after 90 days. Any unused or expired vials must be destroyed by a reverse distributor; for further information, contact your local DEA field office or call Fidelis Animal Health at 1-833-384-4729.Information for Physician:
Ethiqa XR contains a mu opioid partial agonist (1.3 mg buprenorphine/mL). In the case of an emergency, provide the physician with this package insert. Naloxone may not be effective in reversing respiratory depression produced by buprenorphine. The onset of naloxone effect may be delayed by 30 minutes or more. Doxapram hydrochloride has also been used as a respiratory stimulant. -
PRECAUTIONS
The use of paper or soft bedding for up to 3 days following administration of Ethiqa XR should be considered (see CONTRAINDICATIONS and ADVERSE REACTIONS).
Buprenorphine is excreted in the feces (see CLINICAL PHARMACOLOGY). Coprophagy may lead to ingestion of buprenorphine or its metabolites by animals treated with Ethiqa XR and untreated cage mates.
Ethiqa XR forms a depot near the injection site.
Animals may exhibit an obtunded response to stimuli up to 4 hours after receiving Ethiqa XR.
When using Ethiqa XR, an opiate antagonist such as naloxone, should be available in case reversal is required.
Ethiqa XR may cause sedation, decreased blood pressure, decreased heart rate, decreased gastrointestinal mobility, and respiratory depression. Use caution with concomitant administration of Ethiqa XR with drugs that cause respiratory depression.
Animals should be monitored for signs of decreased cardiovascular and respiratory function when receiving Ethiqa XR.
The safety of Ethiqa XR has not been evaluated in pregnant, lactating, neonatal, or immune-compromised animals.
Species-specific precautions described in the published literature are included in the dosing table under the DOSAGE AND ADMINISTRATION section.
- ADVERSE REACTIONS
-
CONTACT INFORMATION
Contact Fidelis Animal Health at 1-833-384-4729 or www.ethiqaxr.com. To report suspected adverse drug experiences, contact Fidelis Animal Health at 1-833-384-4729.
For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
-
CLINICAL PHARMACOLOGY3
Mechanism of Action: Buprenorphine exerts its analgesic effect via high affinity binding to various subclasses of opiate receptors particularly mu, in the central nervous system. Buprenorphine analgesic and adverse reactions are mediated by mu opioid receptor agonism. Due to its partial agonist activity, buprenorphine exhibits a ceiling affect to its actions and thus has a greater therapeutic index compared to full mu opioid receptor agonists such as morphine. Buprenorphine binds tightly to and dissociates slowly from the opioid receptor. Therefore, the pharmacological effects of buprenorphine are not directly related to plasma concentrations.
Buprenorphine can act as an agonist and antagonist at different classes of opioid receptors. Agonism at the mu opioid receptor and, in some cases, antagonism at the kappa or delta opioid receptors are possible underlying mechanisms for the ceiling effect and bell-shaped dose-response curve of buprenorphine. Studies with knockout mice have shown that the antinociceptive effect of buprenorphine, which is mediated primarily by the mu opioid receptor, is attenuated by the ability of the drug to activate the opioid receptor like (ORL-1) receptor. The drug can be described as a ‘full’ and a ‘partial’ agonist at the same receptor depending on the specific assay. There appears to be no ceiling effect for analgesia, but there is a ceiling effect for respiratory depression.
Pharmacokinetic studies with bolus injections of buprenorphine in mice and rats provide similar models. After bolus intravenous administration, plasma levels decline tri-exponentially. The drug is n-dealkylated in the liver to norbuprenorphine (NBN), an active metabolite. Studies have shown that glucuronide metabolites of buprenorphine and NBN are also metabolically active, and can approximate or exceed the concentration of the parent drug. Un-metabolized drug excreted in the urine and feces one week after injection was 1.9 and 22.4% of the dose, respectively, and 92% of the dose was accounted for in one week.3
See the dosing table under DOSAGE AND ADMINISTRATION section for information specific to each species regarding time to reach estimated therapeutic blood levels.
- HOW SUPPLIED
-
STORAGE INFORMATION
Store between 15° and 25°C +/- 2°C (59° and 77°F) or refrigerated. DO NOT FREEZE. If stored refrigerated, bring to room temperature before use. Once broached, the multi-dose vial should be discarded after 90 days.
Product could change its physical properties if not stored within the specified storage conditions and original vial container.
-
REFERENCES
1. Mishra et al. Engineering solid lipid nanaparticles for improved drug delivery: promises and challenges of translational research. Drug Deliv. and Transl. Res, 2: 238-253; 2012.
2. Bethune et al., The role of drug-lipid interactions on the disposition of liposome-formulated opioid analgesics in vitro and in vivo. Anesth Analg. 93(4):928-33; 2001.
3. Guarnieri et al.,Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Animal, 41(11): 337-343; 2012.
4. Levinson BL, Leary SL, Bassett BJ, Cook CJ, GormanGS, Coward LU. Pharmacokinetic and Histopathologic Study of an Extended-Release, Injectable formulation of Buprenorphine in Sprague-Dawley Rats. J Am Assoc Lab Anim Sci. Jan 1, 61(1): 81-8; 2022.
5. Fidelis’ postmarketing surveillance database.
6. Fabian NJ et al. Evaluation and comparison of pharmacokinetic profiles and safety of two extended-release buprenorphine formulations in common marmosets (Callithrix jacchus). Scientific Reports, 13, 11864; 2023.
7. Klein H. et al. A pharmacokinetic study of extended-release buprenorphine in Cynomolgus monkeys (m.fascicularis). Journal of Medical Primatology. 52(6):369-373; 2023.
8. Williams W et al. Pharmacokinetics of sustained-release buprenorphine in adult baboons (Papio Anubis). 2021 National Meeting of the Am Assoc for Lab Anim Sci (virtual).
9. Katzenbach JE, Wittenburg LA, Allweiler SI, Gustafson DL, Johnson MS. Pharmacokinetics of single-dose buprenorphine, butorphanol, and hydromorphone in the domestic ferret (Mustela putorius furo). J Exotic Pet Med 27:95-102; 2018.
10. Chan G et al. Assessment of the Safety and Efficacy of Pre-emptive Use of Extended-release Buprenorphine for Mouse Laparotomy. J Am Assoc Lab Anim Sci 99(99): 1-7;2022.
11. Traul KA et al. Safety studies of post-surgical buprenorphine therapy for mice. Lab Anim. 49(2):100-110;2015.
12. Cowan A et al. Lack of adverse effects during a target animal safety trial of extended-release buprenorphine in Fisher 344 rats. Nature America, Inc. Jan Vol 45(1):28-34; 2016.
13. Bowie AR et al. Pharmacokinetics of Extended-release Buprenorphine in Mongolian Gerbils (Meriones unguiculatus). J Am Assoc Lab Anim Sci: 62 (6): 538-544; 2023.
14. Zanetti AS et al. Pharmacokinetics and adverse effects of 3 sustained-release buprenorphine dosages in healthy guinea pigs. J Am Assoc Lab Anim Sci 56 (6): 768-778; 2017.
15. Fox L et al. Analgesic Efficacy and Safety of Buprenorphine in Chinchillas (Chinchilla lanigera). J Am Assoc Lab Anim Sci 57 (3): 286-290; 2018.
16. Alamaw ED et al. Extended-release Buprenorphine, an FDA indexed Analgesic, Attenuates Mechanical Hypersensitivity in Rats (Rattus norvegicus). J Am Assoc Lab Anim Sci. 61 (1): 81-88; 2022.
17. Oliver VL et al. Evaluation of pain assessment techniques and analgesic efficacy in a female guinea pig model of surgical pain. Am Assoc Lab Anim Med. 56 (4): 425-435; 2017.
18: Hutson CL et al. Analgesia during Monkeypox Virus Experimental Challenge Studies in Prairie Dogs (Cynomys ludovicianus). J Am Assoc Lab Anim Sci. 58 (4):485-500; 2019.
19. Farkas MR, Dorn S, Muller L, et al. Pharmacokinetics, Fecal Output, and Grimace Scores in Rabbits Given Long-Acting Buprenorphine or Fentanyl for Postsurgical Analgesia. J Am Assoc Lab Anim Sci.; 2024.
20. New Zealand white rabbits receiving 0.15 mg/kg and 0.3 mg/kg SQ Ethiqa XR. Unpublished study 2021. Data on file.
21. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 7(2):27-31; 2016.
22. Food and Drug Administration. FDA Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. July 2005. -
SPL UNCLASSIFIED SECTION
MANUFACTURED FOR
Fidelis Animal Health, Inc.
685 US Highway One, Suite 265
North Brunswick, NJ 08902833-384-4729
www.EthiqaXR.comFidelis, Fidelis Animal Health™, Ethiqa XR®, and Fidelipid LAI™ are trademarks of Fidelis Animal Health, Inc., a Delaware Corporation.
NDC 86084-100-30. U.S. Patent Nos. 10,555,899; 11,058,629
FID-ETH-PIR014
WARNING: Due to serious human safety and abuse concerns, read the entire package insert before using this drug, including the complete Boxed Warning.
- Packaging
-
INGREDIENTS AND APPEARANCE
ETHIQA XR
buprenorphine hydrochloride injection, suspension, extended releaseProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:86084-100 Route of Administration SUBCUTANEOUS DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPRENORPHINE HYDROCHLORIDE (UNII: 56W8MW3EN1) (BUPRENORPHINE - UNII:40D3SCR4GZ) BUPRENORPHINE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength CHOLESTEROL (UNII: 97C5T2UQ7J) BENZYL ALCOHOL (UNII: LKG8494WBH) GLYCERYL TRISTEARATE (UNII: P6OCJ2551R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86084-100-30 1 in 1 CARTON 1 3 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date legally marketed unapproved new animal drugs for minor species MIF900014 01/01/2020 Labeler - Fidelis Animal Health, Inc. (080839562)



