Label: AMMONIA N 13- ammonia n-13 injection
- NDC Code(s): 71162-001-05, 71162-001-10
- Packager: Ionetix Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMMONIA N 13 INJECTION safely and effectively. See full prescribing information for AMMONIA N 13 INJECTION. AMMONIA N 13 ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAmmonia N 13 Injection is indicated for diagnostic Positron Emission Tomography (PET) imaging of the myocardium under rest or pharmacologic stress conditions to evaluate myocardial perfusion in ...
-
2 DOSAGE AND ADMINISTRATION2.1 Rest Imaging Study - Aseptically withdraw Ammonia N 13 Injection from its container and administer 10-20 mCi (0.368 – 0.736 GBq) as a bolus through a catheter inserted into a large peripheral ...
-
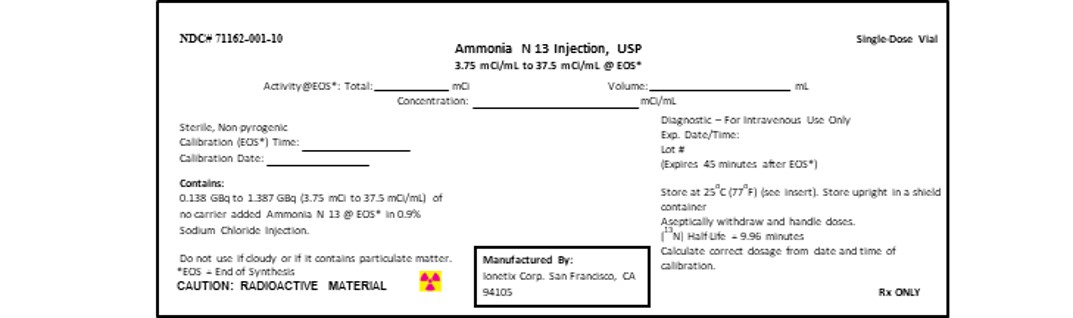
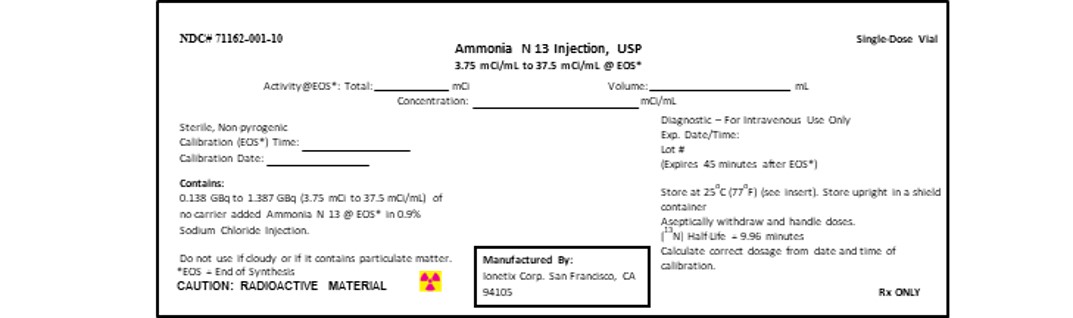
3 DOSAGE FORMS AND STRENGTHSGlass vial (10 mL) or Syringe (5mL) containing 0.138-1.387 GBq (3.75-37.5 mCi/mL) of Ammonia N 13 Injection in aqueous 0.9 % sodium chloride solution (approximately 4 or 6 mL volume).
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Radiation Risks - Ammonia N 13 Injection may increase the risk of cancer. Use the smallest dose necessary for imaging and ensure safe handling to protect the patient and health care worker ...
-
6 ADVERSE REACTIONSNo adverse reactions have been reported for Ammonia N 13 Injection based on a review of the published literature, publicly available reference sources, and adverse drug reaction reporting systems ...
-
7 DRUG INTERACTIONSThe possibility of interactions of Ammonia N 13 Injection with other drugs taken by patients undergoing PET imaging has not been studied.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C - Animal reproduction studies have not been conducted with Ammonia N 13 Injection. It is also not known whether Ammonia N 13 Injection can cause fetal harm ...
-
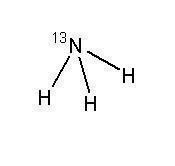
11 DESCRIPTION11.1 Chemical Characteristics - Ammonia N 13 Injection is a positron emitting radiopharmaceutical that is used for diagnostic purposes in conjunction with positron emission tomography (PET ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ammonia N 13 Injection is a radiolabeled analog of ammonia that is distributed to all organs of the body after intravenous administration. It is extracted from the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long term animal studies have not been performed to evaluate the carcinogenic potential of Ammonia N 13 Injection. Genotoxicity assays ...
-
14 CLINICAL STUDIESIn a descriptive, prospective, blinded image interpretation study of adult patients with known or suspected coronary artery disease, myocardial perfusion deficits in stress and rest PET images ...
-
15 REFERENCESAnnals of the ICRP. Publication 53. Radiation dose to patients from radiopharmaceuticals. New York: Pergamon Press, 1988. Demer, L.L.K.L.Gould, R.A.Goldstein, R.L.Kirkeeide, N.A.Mullani, R.W ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAmmonia N 13 Injection is a clear and colorless solution packaged in 10 mL single dose glass vial containing between 0.8325 GBq to 8.325 GBq (22.5 mCi to 225 mCi) or in a 5 mL single dose syringe ...
-
17 PATIENT COUNSELING INFORMATION17.1 Pre-study Hydration - Instruct patients to drink plenty of water or other fluids (as tolerated) in the 4 hours before their PET study. 17.2 Post-study Voiding - Instruct patients to void ...
-
SPL UNCLASSIFIED SECTIONManufactured and Distributed by: IONETIX CORPORATION - San Francisco, CA 94105
-
PRINCIPAL DISPLAY PANEL - 3.75-37.5 mCi/mL Vial Container Label5-mL Syringe Label
-
INGREDIENTS AND APPEARANCEProduct Information