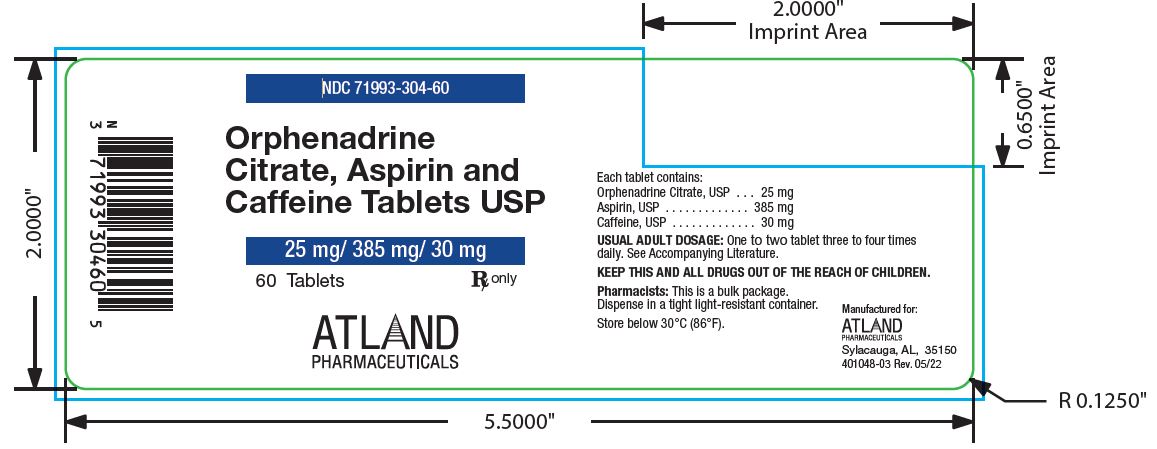
Label: ORPHENADRINE CITRATE, ASPIRIN AND CAFFEINE- orphenadrine citrate, aspirin and caffeine tablet
- NDC Code(s): 71993-304-60
- Packager: Atland Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each Orphenadrine Citrate, Aspirin and Caffeine Tablet, for oral administration contains Orphenadrine Citrate 25 mg, Aspirin 385 mg and Caffeine 30 mg.
In addition, each tablet contains the following inactive ingredients: anhydrous lactose, colloidal silicon dioxide, D&C yellow #10, FD&C blue #1, zinc stearate, povidone, pregelatinized starch, and stearic acid.
Orphenadrine citrate is (2-dimethylaminoethyl 2-methylbenzhydryl ether citrate). It is a white, practically odorless, crystalline powder, having a bitter taste. It is sparingly soluble in water; slightly soluble in alcohol. It has the following structural formula: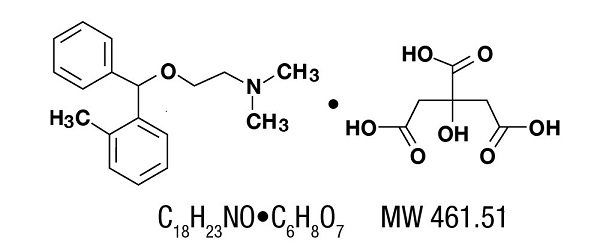
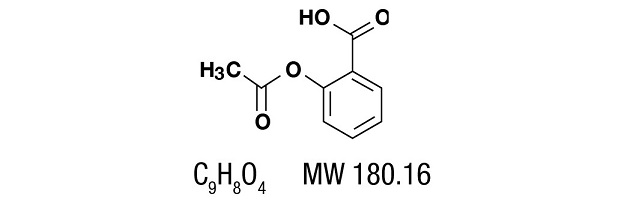
Aspirin, salicylic acid acetate, is a non-opiate analgesic, anti-inflammatory and antipyretic agent It occurs as a white, crystalline tabular or needle-like powder and is odorless or has a faint odor. It is sparingly soluble in water, freely soluble in alcohol and chloroform. It has the following structural formula:
Caffeine is a central nervous system stimulant which occurs as a white powder or white glistening needles, usually matted together. It is sparingly soluble in alcohol, and freely soluble in chloroform. The chemical name for caffeine is, 1,3,7-Trimethylxanthine. It has the following structural formula:
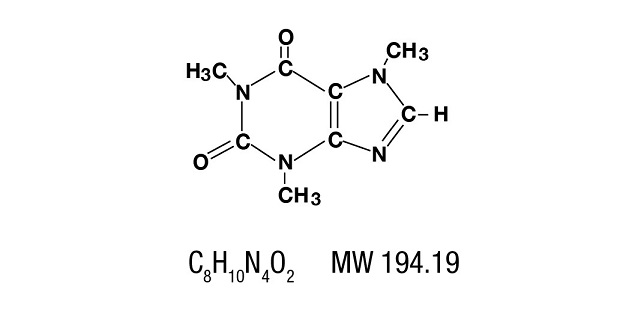
-
CLINICAL PHARMACOLOGY
Orphenadrine citrate is a centrally acting (brain stem) compound which in animals selectively blocks facilitatory functions of the reticular formation. Orphenadrine does not produce myoneural block, nor does it affect crossed extensor reflexes. Orphenadrine prevents nicotine-induced convulsions but not those produced by strychnine.
Chronic administration of Orphenadrine Citrate, Aspirin and Caffeine to dogs and rats has revealed no drug-related toxicity. No blood or urine changes were observed, nor were there any macroscopic or microscopic pathological changes detected. Extensive experience with combinations containing aspirin and caffeine has established them as safe agents. The addition of orphenadrine citrate does not alter the toxicity of aspirin and caffeine.
The mode of therapeutic action of orphenadrine has not been clearly identified, but may be relegated to its analgesic properties. Orphenadrine citrate also possesses anti-cholinergic actions.
-
INDICATIONS & USAGE
Orphenadrine Citrate, Aspirin and Caffeine 25 mg/ 385 mg/ 30 mg Tablets are indicated in:
- Symptomatic relief of mild to moderate pain of acute musculoskeletal disorders.
- The orphenadrine component is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute painful musculoskeletal conditions.
The mode of action of orphenadrine has not been clearly identified, but may be related to its analgesic properties. Orphenadrine Citrate, Aspirin and Caffeine Tablets do not directly relax tense muscles in man.
-
CONTRAINDICATIONS
Because of the mild anticholinergic effect of orphenadrine, Orphenadrine Citrate, Aspirin and Caffeine Tablets should not be used in patients with glaucoma, pyloric or duodenal obstruction, achalasia, prostatic hypertrophy or obstructions at the bladder neck. Orphenadrine Citrate, Aspirin and Caffeine Tablets are also contraindicated in patients with myasthenia gravis and in patients known to be sensitive to aspirin or caffeine.
The drug is contraindicated in patients who have demonstrated a previous hypersensitivity to the drug.
-
WARNINGS
Reye’s Syndrome may develop in individuals who have chicken pox, influenza, or flu symptoms. Some studies suggest possible association between the development of Reye’s Syndrome and the use of medicines containing salicylate or aspirin. Orphenadrine Citrate, Aspirin and Caffeine Tablets 25 mg/ 385 mg/ 30 mg contain aspirin and therefore are not recommended for use in patients with chicken pox, influenza, or flu symptoms.
Orphenadrine Citrate, Aspirin and Caffeine Tablets may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle; ambulatory patients should therefore be cautioned accordingly.
Aspirin should be used with extreme caution in the presence of peptic ulcers and coagulation abnormalities.
Pregnancy
Risk Summary
Use of NSAIDs, including aspirin, can cause premature closure of the fetal ductus arteriosus and fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. Because of these risks, limit dose and duration of Orphenadrine Citrate, Aspirin and Caffeine Tablets use between about 20 and 30 weeks of gestation, and avoid Orphenadrine Citrate, Aspirin and Caffeine Tablets use at about 30 weeks of gestation and later in pregnancy [ see WARNINGS; Fetal Toxicity].
Premature Closure of Fetal Ductus Arteriosus
Use of NSAIDs, including aspirin, at about 30 weeks gestation or later in pregnancy increases the risk of premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment
Use of NSAIDs at about 20 weeks gestation or later in pregnancy has been associated with cases of fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment.
Data from observational studies regarding other potential embryofetal risks of NSAID use in women in the first or second trimesters of pregnancy are inconclusive.
Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation, and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as aspirin, resulted in increased pre- and post-implantation loss. Prostaglandins also have been shown to have an important role in fetal kidney development. In published animal studies, prostaglandin synthesis inhibitors have been reported to impair kidney development when administered at clinically relevant doses.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Premature Closure of Fetal Ductus Arteriosus: Avoid use of NSAIDs in women at about 30 weeks gestation and later in pregnancy, because NSAIDs, including Orphenadrine Citrate, Aspirin and Caffeine Tablets, can cause premature closure of the fetal ductus arteriosus (see WARNINGS; Fetal Toxicity) .
Oligohydramnios/Neonatal Renal Impairment
If an NSAID is necessary at about 20 weeks gestation or later in pregnancy, limit the use to the lowest effective dose and shortest duration possible. If Orphenadrine Citrate, Aspirin and Caffeine Tablets treatment extends beyond 48 hours, consider monitoring with ultrasound for oligohydramnios. If oligohydramnios occurs, discontinue Orphenadrine Citrate, Aspirin and Caffeine Tablets and follow up according to clinical practice (see WARNINGS; Fetal Toxicity) .
Data
Human Data
Premature Closure of Fetal Ductus Arteriosus:
Published literature reports that the use of NSAIDs at about 30 weeks of gestation and later in pregnancy may cause premature closure of the fetal ductus arteriosus.
Oligohydramnios/Neonatal Renal Impairment:
Published studies and postmarketing reports describe maternal NSAID use at about 20 weeks gestation or later in pregnancy associated with fetal renal dysfunction leading to oligohydramnios, and in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. In many cases, but not all, the decrease in amniotic fluid was transient and reversible with cessation of the drug. There have been a limited number of case reports of maternal NSAID use and neonatal renal dysfunction without oligohydramnios, some of which were irreversible. Some cases of neonatal renal dysfunction required treatment with invasive procedures, such as exchange transfusion or dialysis.
Methodological limitations of these postmarketing studies and reports include lack of a control group; limited information regarding dose, duration, and timing of drug exposure; and concomitant use of other medications. These limitations preclude establishing a reliable estimate of the risk of adverse fetal and neonatal outcomes with maternal NSAID use. Because the published safety data on neonatal outcomes involved mostly preterm infants, the generalizability of certain reported risks to the full-term infant exposed to NSAIDs through maternal use is uncertain.
Usage in Children:
The safe and effective use of this drug in children has not been established. Usage of this drug in children under 12 years of age is not recommended.
Fetal Toxicity
Premature Closure of Fetal Ductus Arteriosus:
Avoid use of NSAIDs, including Orphenadrine Citrate, Aspirin and Caffeine Tablets, in pregnant women at about 30 weeks gestation and later. NSAIDs including Orphenadrine Citrate, Aspirin and Caffeine Tablets, increase the risk of premature closure of the fetal ductus arteriosus at approximately this gestational age.
Oligohydramnios/Neonatal Renal Impairment:
Use of NSAIDs, including Orphenadrine Citrate, Aspirin and Caffeine Tablets, at about 20 weeks gestation or later in pregnancy may cause fetal renal dysfunction leading to oligohydramnios and, in some cases, neonatal renal impairment. These adverse outcomes are seen, on average, after days to weeks of treatment, although oligohydramnios has been infrequently reported as soon as 48 hours after NSAID initiation. Oligohydramnios is often, but not always, reversible with treatment discontinuation. Complications of prolonged oligohydramnios may, for example, include limb contractures and delayed lung maturation. In some postmarketing cases of impaired neonatal renal function, invasive procedures such as exchange transfusion or dialysis were required.
If NSAID treatment is necessary between about 20 weeks and 30 weeks gestation, limit Orphenadrine Citrate, Aspirin and Caffeine Tablets use to the lowest effective dose and shortest duration possible. Consider ultrasound monitoring of amniotic fluid if Orphenadrine Citrate, Aspirin and Caffeine Tablets treatment extends beyond 48 hours. Discontinue Orphenadrine Citrate, Aspirin and Caffeine Tablets if oligohydramnios occurs and follow up according to clinical practice [ see PRECAUTIONS; Pregnancy] .
Serious Skin Reactions
NSAIDs, including aspirin, a component of Orphenadrine Citrate, Aspirin and Caffeine Tablets, can cause serious skin adverse reactions such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. NSAIDs can also cause fixed drug eruption (FDE). FDE may present as a more severe variant known as generalized bullous fixed drug eruption (GBFDE), which can be life-threatening. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin reactions, and to discontinue the use of Orphenadrine Citrate, Aspirin and Caffeine Tablets at the first appearance of skin rash or any other sign of hypersensitivity. Orphenadrine Citrate, Aspirin and Caffeine Tablets is contraindicated in patients with previous serious skin reactions to NSAIDs [ see Contraindications].Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as Orphenadrine Citrate, Aspirin and Caffeine Tablets. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, discontinue Orphenadrine Citrate, Aspirin and Caffeine Tablets and evaluate the patient immediately.
-
PRECAUTIONS
Confusion, anxiety and tremors have been reported in a few patients receiving propoxyphene and orphenadrine concomitantly. As these symptoms may be simply due to an additive effect, reduction of dosage and/or discontinuation of one or both agents is recommended in such cases.
Safety of continuous long term therapy with Orphenadrine Citrate, Aspirin and Caffeine Tablets has not been established; therefore, if Orphenadrine Citrate, Aspirin and Caffeine Tablets are prescribed for prolonged use, periodic monitoring of blood, urine and liver function values is recommended.
Pregnancy
Embryo-Fetal Toxicity
Inform pregnant women to avoid use of aspirin and other NSAIDs starting at 30 weeks gestation because of the risk of the premature closing of the fetal ductus arteriosus. If treatment with Orphenadrine Citrate, Aspirin and Caffeine Tablets is needed for a pregnant woman between about 20 to 30 weeks gestation, advise her that she may need to be monitored for oligohydramnios, if treatment continues for longer than 48 hours [ see WARNINGS; Fetal Toxicity, PRECAUTIONS; Pregnancy] .
Serious Skin Reactions, including DRESS
Advise patients to stop taking Orphenadrine Citrate, Aspirin and Caffeine Tablets immediately if they develop any type of rash or fever and to contact their healthcare provider as soon as possible [ see Warnings].
-
ADVERSE REACTIONS
Side effects of Orphenadrine Citrate, Aspirin and Caffeine Tablets are those seen with aspirin and caffeine or those usually associated with mild anti-cholinergic agents. These may include tachycardia, palpitation, urinary hesitancy or retention, dry mouth, blurred vision, dilation of the pupil, increased intraocular tension, weakness, nausea, vomiting, headache, dizziness, constipation, drowsiness, and rarely, urticaria and other dermatosis. Infrequently, an elderly patient may experience some degree of confusion. Mild central excitation and occasional hallucinations may be observed. These mild side effects can usually be eliminated by reduction in dosage. One case of aplastic anemia associated with the use of orphenadrine citrate, aspirin and caffeine has been reported. No causal relationship has been established. Rare G.I. hemorrhage due to aspirin content may be associated with the administration of Orphenadrine Citrate, Aspirin and Caffeine Tablets. Some patients may experience transient episodes of light-headedness, dizziness or syncope.
Orphenadrine Citrate, Aspirin and Caffeine Tablets may also cause exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), toxic epidermal necrosis (TEN), and fixed drug eruption (FDE) ( see WARNINGS).
- DOSAGE & ADMINISTRATION
-
HOW SUPPLIED
Orphenadrine Citrate, Aspirin, and Caffeine Tablets (Orphenadrine Citrate 25 mg, Aspirin 385 mg, and Caffeine 30 mg): Two-layered, white/green round flat faced beveled edge tablet debossed “OAC” over “472” on the white side and plain on the green side. They are available in bottles of 60 tablets (NDC 71993-304-60).
Store below 30°C (86°F)
Rx Only
Manufactured for:
ATLAND Pharmaceuticals, LLC
Sylacauga, AL 35150500534-02
Rev. 07/2024 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ORPHENADRINE CITRATE, ASPIRIN AND CAFFEINE
orphenadrine citrate, aspirin and caffeine tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71993-304 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ORPHENADRINE CITRATE (UNII: X0A40N8I4S) (ORPHENADRINE - UNII:AL805O9OG9) ORPHENADRINE CITRATE 25 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 385 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 30 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ZINC STEARATE (UNII: H92E6QA4FV) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score no score Shape ROUND Size 11mm Flavor Imprint Code OAC472 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71993-304-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/18/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075141 03/18/2022 Labeler - Atland Pharmaceuticals, LLC (080942150)