Label: ADRENALIN (EPINEPHRINE IN SODIUM CHLORIDE)- epinephrine in sodium chloride injection
- NDC Code(s): 42023-315-01, 42023-315-10
- Packager: Endo USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADRENALIN safely and effectively. See full prescribing information for ADRENALIN. ADRENALIN (epinephrine in sodium chloride ...
-
Table of ContentsTable of Contents
-
1. INDICATIONS AND USAGE
1.1. Hypotension - associated with Septic Shock - Adrenalin is indicated to increase mean arterial blood pressure in adult patients with hypotension associated with septic shock.
-
2. DOSAGE AND ADMINISTRATION
2.1. General Considerations - Administration - Adrenalin is a ready to administer product that requires no further dilution prior to infusion. Inspect visually for particulate matter and ...
-
3. DOSAGE FORMS AND STRENGTHS
Injection: Epinephrine in sodium chloride is a clear, colorless solution in a ready-to-use, single-dose container available as: 2 mg/250mL (8 mcg/mL) 4 mg/250mL (16 mcg/mL) 5 mg/250mL (20 ...
-
4. CONTRAINDICATIONS
None.
-
5. WARNINGS AND PRECAUTIONS
5.1. Hypertension - Because individual response to epinephrine may vary significantly, monitor blood pressure frequently and titrate to avoid excessive increases in blood pressure. Patients ...
-
6. ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in labeling: Hypertension [see Warnings and Precautions (5.1)] Pulmonary Edema [see Warnings and Precautions (5.2)] Cardiac Arrhythmias ...
-
7. DRUG INTERACTIONS
7.1. Drugs Antagonizing Pressor Effects of Epinephrine - α-blockers, such as phentolamine - Vasodilators, such as nitrates - Diuretics - Antihypertensives - Ergot alkaloids - Phenothiazine ...
-
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy - Risk Summary - Limited published data on epinephrine use in pregnant women are not sufficient to determine a drug-associated risk of major birth defects or miscarriage ...
-
10. OVERDOSAGE
Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in ...
-
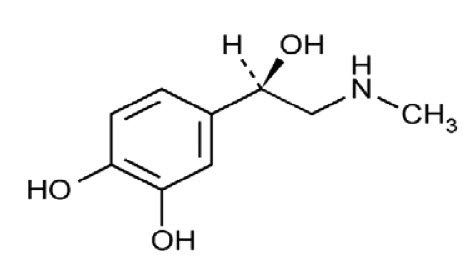
11. DESCRIPTION
Adrenalin (epinephrine in sodium chloride injection) is a sympathomimetic catecholamine. The chemical name of epinephrine is: 1,2- Benzenediol, 4-[(1R)-1-hydroxy-2-(methylamino)ethyl]-, or ...
-
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action - Epinephrine acts on both alpha and beta-adrenergic receptors. The mechanism of the rise in blood pressure is 3-fold: a direct myocardial stimulation that increases ...
-
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies to evaluate the carcinogenic potential of epinephrine have not been conducted. Epinephrine and other ...
-
16. HOW SUPPLIED/STORAGE AND HANDLING
Adrenalin (epinephrine in sodium chloride injection) is supplied as a clear, colorless sterile solution in a single-dose 250 mL non-PVC infusion bag with a single function connector system ...
-
PRINCIPAL DISPLAY PANEL - 4 mg per 250 mL (16 mcg/mL)

-
INGREDIENTS AND APPEARANCEProduct Information