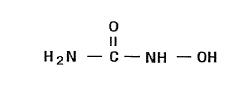Label: HYDREA- hydroxyurea capsule
- NDC Code(s): 61269-835-10
- Packager: H2-Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use HYDREA safely and effectively. See full prescribing information for HYDREA. HYDREA (hydroxyurea) capsules, for oral use - Initial ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEHYDREA is indicated for the treatment of: • Resistant chronic myeloid leukemia. • Locally advanced squamous cell carcinomas of the head and neck (excluding the lip) in combination with ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - HYDREA is used alone or in conjunction with other antitumor agents or radiation therapy to treat neoplastic diseases. Individualize treatment based on tumor type, disease ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 500 mg opaque green cap and opaque pink body imprinted with "HYDREA" and "500".
-
4 CONTRAINDICATIONSHYDREA is contraindicated in patients who have demonstrated a previous hypersensitivity to hydroxyurea or any other component of the formulation.
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - Hydroxyurea causes severe myelosuppression. Treatment with HYDREA should not be initiated if bone marrow function is markedly depressed. Bone marrow suppression may occur ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described in detail in other labeling sections: Myelosuppression [see Warnings and Precautions (5.1)] Hemolytic anemia [see Warnings ...
-
7 DRUG INTERACTIONS7.1 Increased Toxicity with Concomitant Use of Antiretroviral Drugs - Pancreatitis - In patients with HIV infection during therapy with hydroxyurea and didanosine, with or without stavudine ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - HYDREA can cause fetal harm based on findings from animal studies and the drug's mechanism of action [see Clinical Pharmacology (12.1)]. There are no data with ...
-
10 OVERDOSAGEAcute mucocutaneous toxicity has been reported in patients receiving hydroxyurea at dosages several times the therapeutic dose. Soreness, violet erythema, edema on palms and soles followed by ...
-
11 DESCRIPTIONHYDREA (hydroxyurea capsules, USP) is an antimetabolite available for oral use as capsules containing 500 mg hydroxyurea. Inactive ingredients include citric acid, colorants (D&C Yellow No. 10 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The precise mechanism by which hydroxyurea produces its antineoplastic effects cannot, at present, be described. However, the reports of various studies in tissue ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Conventional long-term studies to evaluate the carcinogenic potential of hydroxyurea have not been performed. However, intraperitoneal ...
-
15 REFERENCESOSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html.
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - HYDREA® (hydroxyurea capsules, USP) is supplied as 500 mg capsules in HDPE bottles with a plastic safety screw cap. Each bottle contains 100 capsules. The cap is opaque green ...
-
17 PATIENT COUNSELING INFORMATIONThere is a risk of myelosuppression. Monitoring blood counts weekly throughout the duration of therapy should be emphasized to patients taking HYDREA. Advise patients to report signs and symptoms ...
-
PRINCIPAL DISPLAY PANEL - 500 mg Capsule Bottle Label100 CAPSULES - NDC 61269-835-10 - HYDREA® (hydroxyurea) capsules - 500 mg - CAUTION: Cytotoxic Agent - CHEPLAPHARM
-
PRINCIPAL DISPLAY PANEL - 500 mg Capsule Bottle Carton100 CAPSULES - NDC 61269-835-10 - HYDREA® (hydroxyurea) capsules - 500 mg - CAUTION: Cytotoxic Agent - CHEPLAPHARM
-
INGREDIENTS AND APPEARANCEProduct Information