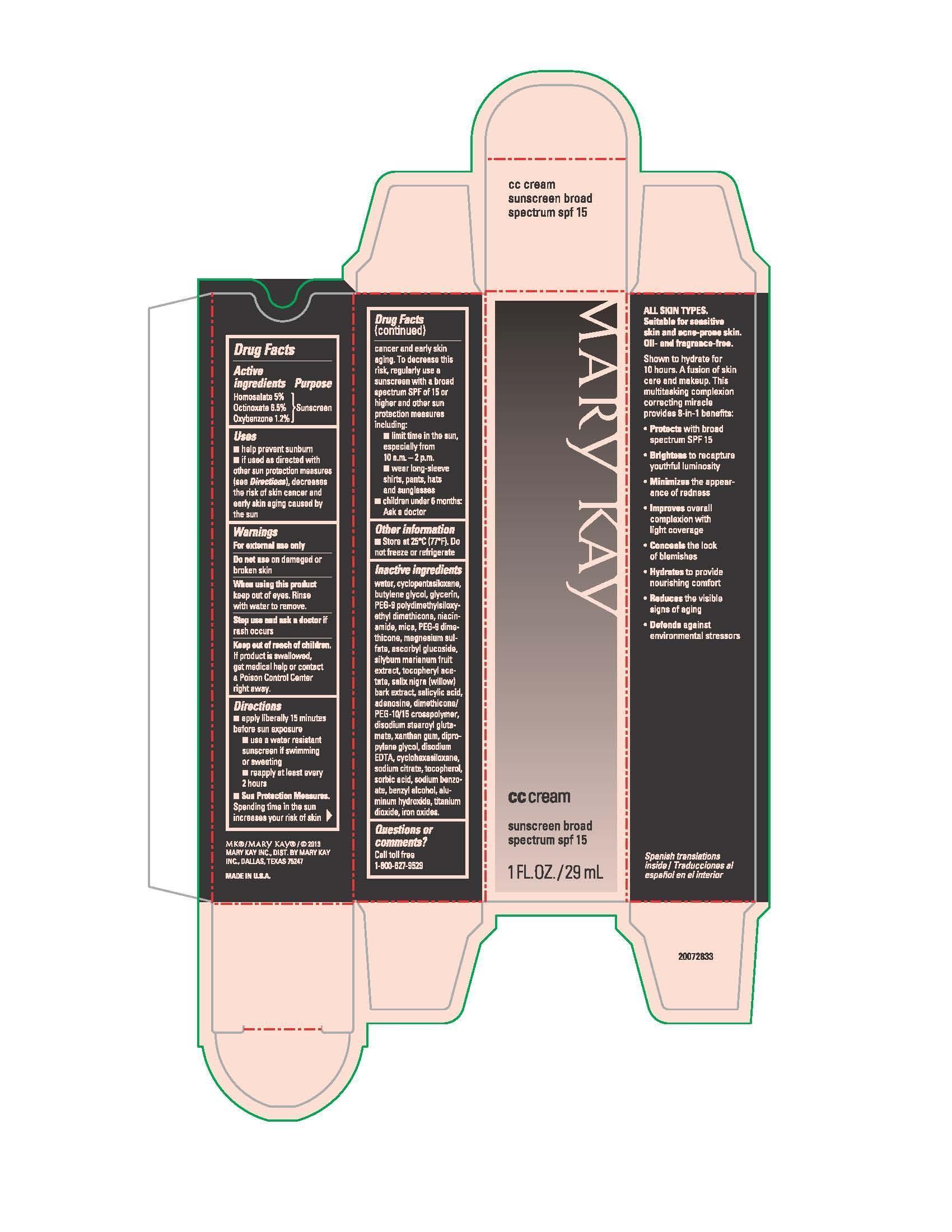
Label: MARY KAY CC CREAM SUNSCREEN BROAD SPECTRUM SPF 15 MEDIUM TO DEEP- homosalate, octinoxate, oxybenzone cream
- NDC Code(s): 51531-2824-1, 51531-2824-3
- Packager: Mary Kay Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats and sunglasses
- children under 6 months: Ask a doctor
- Other information
-
Inactive ingredients
water, cyclopentasiloxane, butylene glycol, glycerin, PEG-9 polydimethylsiloxyethyl dimethicone, niacinamide, mica, PEG-9 dimethicone, magnesium sulfate, ascorbyl glucoside, silybum marianum fruit extract, tocopheryl acetate, salix nigra (willow) bark extract, salicylic acid, adenosine, dimethicone/PEG-10/15 crosspolymer, disodium stearoyl glutamate, xanthan gum, dipropylene glycol, disodium EDTA, cyclohexasiloxane, sodium citrate, tocopherol, sorbic acid, sodium benzoate, benzyl alcohol, aluminum hydroxide, titanium dioxide, iron oxides
- Questions or comments?
- Principal Display Panel - 29 mL carton
-
INGREDIENTS AND APPEARANCE
MARY KAY CC CREAM SUNSCREEN BROAD SPECTRUM SPF 15 MEDIUM TO DEEP
homosalate, octinoxate, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51531-2824 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 5 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.5 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 1.2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) NIACINAMIDE (UNII: 25X51I8RD4) MICA (UNII: V8A1AW0880) PEG-9 DIMETHICONE (400 CST) (UNII: 9OZ27X065D) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) MILK THISTLE (UNII: U946SH95EE) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SALIX NIGRA BARK (UNII: QU52J3A5B3) SALICYLIC ACID (UNII: O414PZ4LPZ) ADENOSINE (UNII: K72T3FS567) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) XANTHAN GUM (UNII: TTV12P4NEE) DIPROPYLENE GLYCOL (UNII: E107L85C40) EDETATE DISODIUM (UNII: 7FLD91C86K) CYCLOMETHICONE 6 (UNII: XHK3U310BA) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) TOCOPHEROL (UNII: R0ZB2556P8) SORBIC ACID (UNII: X045WJ989B) SODIUM BENZOATE (UNII: OJ245FE5EU) BENZYL ALCOHOL (UNII: LKG8494WBH) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIMETHICONE/PEG-10/15 CROSSPOLYMER (UNII: 21AS8B1BSS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51531-2824-1 1 in 1 CARTON 02/16/2014 1 29 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:51531-2824-3 1 mL in 1 PACKET; Type 0: Not a Combination Product 02/16/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/16/2014 Labeler - Mary Kay Inc. (049994452) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 103978839 manufacture(51531-2824) Establishment Name Address ID/FEI Business Operations Englewood Lab Inc. 172198223 manufacture(51531-2824)